��Ŀ����
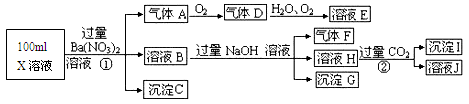
�ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʡ������������������±���ʾ��
| ������ | NH4+��Na����Mg2�� |
| ������ | OH����NO3����SO42�� |
ȡ�����������ֻ��������Ƴ���ͬ�������Һ�������ʵ����ʵ���Ũ�ȣ�c(��)��c(��)��c(��)��
(1)����________����AlCl3��Һ����μ������Һ���۲쵽��������________����Ӧ�����ӷ���ʽΪ________��
(2)����______�����ʵ��ȷ���ҵ��������________(������ȷ��������ÿ�)��
(1)NaOH�������ɰ�ɫ�����������μӣ���ɫ������ʧ��Al3����3OH��=Al(OH)3����Al(OH)3��OH��=AlO2����2H2O
(2)(NH4)2SO4��NH4NO3��ȡ����Һ��С�Թ��У���������BaCl2��Һ�����а�ɫ����������������(NH4)2SO4��������������������NH4NO3
����

 �Ķ��쳵ϵ�д�
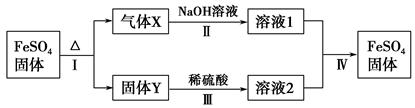
�Ķ��쳵ϵ�д�ij������Һ�п��ܺ���SO42-��SO32-��CO32-��HCO3-��NO3-��Cl����Br���е������ּ�һ�ֳ�������������(Mn��)���ֽ�������ʵ��(ÿ��ʵ�������Լ����������ģ�������ijЩ�ɷֿ���û�и���)��
��ش��������⣺
(1)����������ͼ��Ϣ��д�±�(����ȷ���IJ���)��
| | �϶����ڵ����� | �϶�û�е����� | ����D | |
| ��ѧʽ�����ӷ��� | | | | |
(2)������Һ���Ƿ���SO32-��SO42- ��������D���������ɫ�������ɳ���Dʱ�϶������ķ�Ӧ�����ӷ���ʽΪ ���γɳ���Bʱ��Ӧ�����ӷ���ʽΪ ��
(3)��Mn��Ϊ����������������ԭ������������20����Ҫȷ���������Ǻ������ӵķ����� ��
��.�������(K2FeO4)�Ǽ��õ������������и�Ч���������ã�Ϊһ�����ͷ��ȸ�Ч�������������������������£�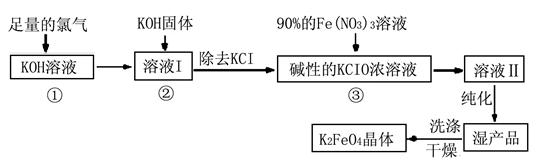
��ͬ���������⡣
��1��д����KOH��Һ��ͨ������Cl2������Ӧ�����ӷ���ʽ ��
��2������ҺI�м���KOH�����Ŀ���� (ѡ�����)��
| A��Ϊ��һ����Ӧ�ṩ���ԵĻ��� |
| B��ʹKClO3ת��ΪKClO |
| C������ҺI�й�����Cl2������Ӧ�����ɸ����KClO |
| D��KOH�����ܽ��ų��϶����������������߷�Ӧ���ʺ�KClO�Ĵ��� |
��4�����������һ�����Ͷ��ε�أ����ҺΪ����Һ���䷴ӦʽΪ��
3Zn(OH)2+2Fe(OH)3+4KOH
 3Zn+2K2FeO4+8H2O��
3Zn+2K2FeO4+8H2O���ŵ�ʱ��صĸ�����ӦʽΪ ��
��.��(N2H4)�ֳ���������һ�ֿ�ȼ�Ե�Һ�壬�������ȼ�ϡ�
��5��д���·��ӵĵ���ʽ ��
��6��������N2O4��Ӧ��2N2H4(g)��N2O4(g)=3N2(g)��4H2O(g) ��H����1076.7 kJ/mol��
��֪��N2(g)��2O2(g)=N2O4(g)����H����8.7 kJ/mol�� д������O2��Ӧ����N2��H2O(g)���Ȼ�ѧ����ʽ ��
��ҵ��ˮ�г�����һ������Cr2O72����CrO42�������ǻ�����༰��̬ϵͳ�����ܴ���˺���������д������÷��Ĺ�������Ϊ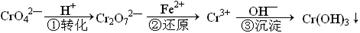
���еڢٲ�����ƽ�⣺2CrO42������ɫ��+2H+ Cr2O72������ɫ��+H2O
Cr2O72������ɫ��+H2O
��1����ƽ����ϵ��pH=2������Һ�� ɫ��
��2����˵���ڢٲ���Ӧ��ƽ��״̬���� ����ѡ���ţ�
| A��Cr2O72����CrO42����Ũ����ͬ | B��v��(Cr2O72��) ="2v" ��(CrO42��) |
| C����Һ����ɫ���� | D����Һ��pHֵ���� |
��4����Cr2(SO4)3��Һ�У��μ�NaOH����pH��4.6ʱ����ʼ����Cr(OH)3����������pH�����ߣ��������࣬����pH��13ʱ��������ʧ����������ɫ��[Cr(OH)4]�����ӡ���ƽ���ϵ���£�
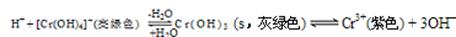
����0.05mol��L��1��Cr2(SO4)3��Һ50mL�У���������0.6 mol��L��1��NaOH��Һ����ַ�Ӧ����Һ�пɹ۲쵽������Ϊ ����Һ������Ũ���ɴ�С��˳��Ϊ ��
��5����Na[Cr(OH)4]��Na2Cr2O7��Ϻ����Һ�м���H2SO4�ữ����Ԫ���� ��ʽ���ڣ���д���ӷ��ţ���
�������ȣ�ClO2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч����������������һ�ֻ���ɫ�����壬������ˮ��
��.��1��ClO2����KClO3��H2SO4���ڵ���������Na2SO3��Ӧ�Ƶá���÷�Ӧ�����������뻹ԭ��������ʵ���֮����________��
��.ʵ����Ҳ����NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ���Ʊ�ClO2�����������£�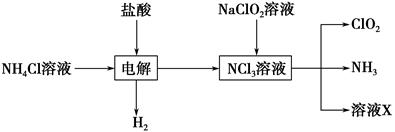
��2��д�����ʱ������Ӧ�Ļ�ѧ����ʽ��________________________________��
��3����ȥClO2�е�NH3��ѡ�õ��Լ���________��������ţ�
| A������ʳ��ˮ | B����ʯ�� |
| C��Ũ���� | D��ˮ |
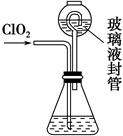
��װ���в���Һ��ܵ�������______________________________________��
����д��������������������⻯����Һ��Ӧ�����ӷ���ʽ__________________________��
�۵ζ��յ��������_______________________________________________��
�ܲ��ͨ��ClO2������m��ClO2����________�����ú�c��V�Ĵ���ʽ��ʾ��
��5����ClO2������������ˮ��pHΪ5.5��6.5��������һ���������岻���������������ClO2-��2001���ҹ��������涨������ˮ��ClO2-����Ӧ������0.2 mg��L��1��������ˮ��ClO2-�ĺ������꣬�������м���������ij��ԭ�����÷�Ӧ������������________���ѧʽ�����䷢����Ӧ�����ӷ���ʽΪ_________________________________________________________________��