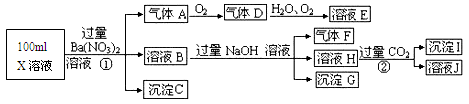
��Ŀ����
ij��ɫ��Һ�к���K+��Cl-��OH-��SO32-��SO42-��Ϊ������Һ�������ĸ��������ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH-��ʵ�鷽�����ԣ��������������ӵĹ�������ͼ��ʾ��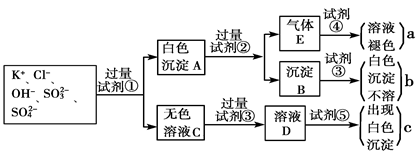
��ش��������⣺
��1����ɫ����A���Լ��ڷ�Ӧ�����ӷ���ʽ��__________________________________��
��2����ɫ��ҺC�м����Լ��۵���ҪĿ����____________________________________��
��3����������ֻ���Լ��۶������Լ��ڣ���ʵ���Ӱ����_______________________��
��4������Eͨ���Լ��ܷ�����Ӧ�����ӷ���ʽ��__________________________________��
��1�� BaSO3 + 2H+  Ba2+ + SO2�� + H2O ��2�֣�
Ba2+ + SO2�� + H2O ��2�֣�
��2�� �к�OH-����ֹ��Cl-�ļ���������ţ�2�֣�
��3�� ��ʹSO32-��SO42-�ļ���������ţ�����ȷ��SO42-��SO32-�Ƿ���ڣ�2�֣�
��4�� SO2 + Br2 + 2H2O  4H+ + SO42- + 2Br-��2�֣�
4H+ + SO42- + 2Br-��2�֣�
������������� ����������Ӻ���������Ӷ������뱵���ӷ�Ӧ���ɰ�ɫ�������������ᱵ����������ˮ�����ܽ������У������ᱵ�����Ȳ��ܽ���ˮ�У�Ҳ���ܽ������С��������������������Һ�лᱻ����Ϊ��������ӣ����Ҫ������������ӵĴ���ʱҪ���ų�������������Ӷ���ĸ��ţ���˼ӵ��Լ�1ӦΪ���ᣬ
���㣺

��ˮ��Һ���ܴ��������һ��������
| A��Al3+��Na+��HCO3����SO42�� | B��H+��Fe2+��ClO����Cl�� |
| C��Mg2+��K+��SO42����NO3�� | D��NH4+��Ag+��OH����Br�� |
���������У����ڷǵ���ʵ���
| A������ | B��NaCl | C������ | D��ͭ |
�������ֱ����뷴Ӧ����ʽ��Ӧ����ȷ����
A���������е���AgNO3��Һ�������е���Ԫ�أ�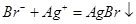 |
B���ô����ȥˮ����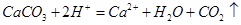 |
C����������Һ��ͨ������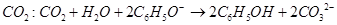 |
D��ʵ������Һ��ͱ��ڴ������������屽�� |
(11�֣�2010�괺��2013���������귢��������ʡ�����غ���ǣ����ȫ��������ģ��������Ƕ�ˮ��Դ���ٴ����ӡ�ˮ��������������Ҫ��ѧ���ʣ��й�ˮ�ķ�Ӧ�кܶࡣ
��1���õ���ʽ��ʾH2O���γɹ��� ��
��2����pH=1��ˮ��Һ�У���NH4+��Al3+��Br-��SO42- �� Na+��Fe2+��Cl-��NO3-
��K+��Ba2+��Cl-��NO3- ��K+��Na+��HCO3-��SO42-���������У�һ������������� (�����)��
��3�������з�Ӧ�У�ˮ�������������� (����ĸ����ͬ)��ˮ�Ȳ����������ֲ�����ԭ������ ��
| A��2F2��2H2O��4HF��O2 | B��2Na2O2��2H2O��4NaOH��O2�� |
| C��CaH2��2H2O��Ca(OH)2��2H2�� | D��3Fe��4H2O Fe3O4��4H2 Fe3O4��4H2 |
��14�֣�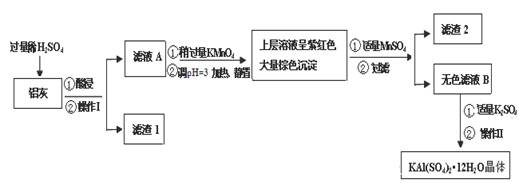
����[KAl(SO4)2��12H2O] ���������������й㷺��;������ˮ�ľ�������ֽ��ҵ����ʩ������ʳƷ��ҵ�ķ��ͼ��ȡ������������ķ��ϡ������ң���Al �� Al2O3������SiO2��FeO ��xFe2O3�����Ʊ������������������£�
�ش��������⣺
��1��������ˮ��ԭ���ǣ������ӷ��̱�ʾ��
��2���������� ��������������Ũ���� �����ˡ� ���
��3��������ҺA���Ƿ����Fe2+�ķ����� ��ֻ��һ���Լ���
��4������ҺA�м��������ص�Ŀ���� ��������Ӧ�����ӷ���ʽΪ����������Fe2+ת��ΪFe3+��MnO4- ת��ΪMn2+�� ��
��֪�������������������pH���±���ʾ
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
���ݱ������ݽ��͵�pH=3��Ŀ�� ��
��5����֪:��pH=3�����������£�MnO4- ����Mn2+��Ӧ����MnO2������MnSO4������Ӧ�����ӷ���ʽΪ�� ������2���е������� ��
��6����Al��NiO(OH)Ϊ�缫��KOH��ҺΪ���Һ��������͡���Ч��أ���ŵ�����У�����Ni(OH)2��NiO(OH)֮���ת����д���ŵ�ʱ��ط�Ӧ�Ļ�ѧ����ʽ ��
ij������Һ�п��ܺ���SO42-��SO32-��CO32-��HCO3-��NO3-��Cl����Br���е������ּ�һ�ֳ�������������(Mn��)���ֽ�������ʵ��(ÿ��ʵ�������Լ����������ģ�������ijЩ�ɷֿ���û�и���)��
��ش��������⣺
(1)����������ͼ��Ϣ��д�±�(����ȷ���IJ���)��
| | �϶����ڵ����� | �϶�û�е����� | ����D | |
| ��ѧʽ�����ӷ��� | | | | |
(2)������Һ���Ƿ���SO32-��SO42- ��������D���������ɫ�������ɳ���Dʱ�϶������ķ�Ӧ�����ӷ���ʽΪ ���γɳ���Bʱ��Ӧ�����ӷ���ʽΪ ��
(3)��Mn��Ϊ����������������ԭ������������20����Ҫȷ���������Ǻ������ӵķ����� ��