��Ŀ����
4��ʵ������Ҫ0.1mol/L NaOH��Һ450mL��0.5mol/L������Һ500mL��������������Һ����������ش��������⣺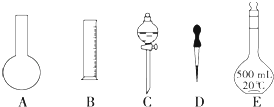
����ͼ��ʾ�������У�������Һ�϶�����Ҫ����AC ������ţ�������������Һ�����õ��IJ����������ձ��������� �����������ƣ���
������0.1mol/L NaOH��Һʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�FEDCBA��
A��������ƿ�ǽ�����ҡ��
B�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
C������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
D����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ
E�����ܽ������������Һ�ز�����ע��500mL������ƿ��
F��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
������0.1mol/L NaOH��Һʱ����ʵ����������������ȷ��������ʱ��������ƿ�̶��ߣ���������ҺŨ��С��0.1mol/L������ڡ��������ڡ���С�ڡ�����
������0.5mol/L������Һ500mLʱ��������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ13.6mL������������һλС������
������0.5mol/L������Һʱ����ʵ����������������ȷ��������Ͳ��ȡŨ����ʱ���ӿ̶��ߣ���������ҺŨ�ȴ���0.5mol/L������ڡ��������ڡ���С�ڡ�����
���� ������������Һ���ѡ����ʵ�����ƿ������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ��������������
�ڸ�������һ�����ʵ���Ũ����Һ�IJ�����ʵ�鲽������
�۸��ݹ�ʽc=$\frac{n}{V}$�����Ը���Ӱ��n��V��������������������
������c=$\frac{1000�Ѧ�}{M}$������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������ʵ���Ũ�ȣ�������Һϡ�������������ʵ����ʵ������������ҪŨ���������
������Ͳ��ȡŨ����ʱ���ӿ̶��ߵ���Ũ��������ƫ��
��� �⣺����Ҫ0.1mol/L NaOH��Һ450mL��ʵ����û��450mL����ƿ��Ӧѡ��500mL����ƿ��
����һ�����ʵ���Ũ����Һ�IJ���˳���ǣ��������������ȡ�����ܽ⣨ϡ�ͣ���ϡ��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����������ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�������Ҫ������Ϊ��������ƽ����Ͳ����ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
�϶�����Ҫ���ǣ�ƽ����ƿ����Һ©������ȱ�ٵ��������ձ�����������
�ʴ�Ϊ��AC���ձ�����������
������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ��˳��Ϊ��FEDCBA��
�ʴ�Ϊ��FEDCBA��
��������ʱ���ӿ̶��ߣ�������Һ�����ƫ���ɹ�ʽc=$\frac{n}{V}$������������Һ��Ũ��ƫ�ͣ���������ҺŨ��С��0.1mol/L���ʴ�Ϊ��С�ڣ�
����������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ�������ʵ���Ũ��c=$\frac{1000��1.84��98%}{98}$=18.4mol/L������ҪŨ�������ΪV����������Һϡ�������������ʵ����ʵ�������ã�V��18.4mol/L=0.5mol/L��0.5L
���V=0.0136L����13.6mL��
�ʴ�Ϊ��13.6��
������Ͳ��ȡŨ����ʱ���ӿ̶��ߵ���Ũ��������ƫ����ȡ��������ʵ���ƫ������c=$\frac{n}{V}$����֪��Һ��Ũ��ƫ�ߣ�����������ҺŨ�ȴ���0.5mol/L��
�ʴ�Ϊ�����ڣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������ǽ���ؼ���ע���������ķ����ͼ��ɣ���Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� 1-����鳣�����л���Ӧ���ܼ���ʵ�����Ʊ�1-����飨CH3CH2CH2Br���ķ�Ӧ����Ҫʵ��װ�����£�
1-����鳣�����л���Ӧ���ܼ���ʵ�����Ʊ�1-����飨CH3CH2CH2Br���ķ�Ӧ����Ҫʵ��װ�����£���֪��
��CH3CH2CH2OH+HBr$\stackrel{��}{��}$CH3CH2CH2Br+H2O
��.2CH3CH2CH2OH$��_{140��}^{Ũ����}$��CH3CH2CH2��2O�������ѣ�+H2O
�����õ�������������£�
| ��Է� ������ | �ܶ� /g•mL-1 | �е�/�� | ˮ�� �ܽ��� | |
| ������ | 60 | 0.896 | 97.1 | �� |
| ������ | 102 | 0.74 | 90 | �������� |
| 1-����� | 123 | 1.36 | 71 | ���� |
����A�м���50g��������һ������Ũ���ᡢ�廯��һ����ȣ����뼸����ʯ�����ڱ���69��72���������2Сʱ���������ռ�68��90�����Һ ����̼������Һϴ�����ԣ���Һ�����������ռ�68��76�����Һ���õ�����1-�����66g����ش�
��1��Bװ�������ǣ����Σ������ܣ���ʯ�������Ƿ�ֹҺ�屩�У�
��2������Aǰ�����ȴ�b���a����b��������B��ͨ��ˮ��
��3������ܵ�Ŀ����Ҫ��ϴȥŨ���ᣬ��ҡ���ã��ֲ���Ӧ�ӷ�Һ©�����£���ϡ����¡����ڷ������
��4����ʵ�����õ���1-����������C������ȷ����ţ���
A.41%������B.50%������C.64%������D.70%
 �屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�
�屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�����1����a�м���15mL����������м���ٽ�b��4.0mLҺ���������뵽a�У���ַ�Ӧ��
����2����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����3����Һ������10mLˮ��8mL 10%��NaOH��Һ��10mL ˮϴ�ӣ�
��Һ�ô��屽��
| �� | �� | �屽 | |
| �ܶ�/gcm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ��ˮ�е��ܽ�� | �� | �� | �� |
��1������1����a�з�������Ҫ��Ӧ��
 ��
����2������c�������������ܣ�����d������������HBr����Ⱦ����������
��3����b�е�Һ���������뵽a�У������ܿ��ټ����ԭ���Ƿ�ֹ��Ӧ�ų�����ʹC6H6��Br2�ӷ���Ӱ����ʣ�
��4������c��������������������������Ҫ������C6H6��Br2���ѧʽ����
��5������4�õ��Ĵֲ�Ʒ�л��������ʱ�����֪�����屽���й������������ϱ�����Ҫ��һ���ᴿ�ֲ�Ʒ����������е�ʵ���������������
��6��ʵ������������ʵ�鷽������dװ��������Һ�к���Br-��
ʵ�������ȡ����d����Һ���Թ��У��μӹ���ϡHNO3���ټ���AgNO3��Һ��
ʵ������ͽ��ۣ��е���ɫ�������ɣ���Һ�к���Br-��
| A�� | ��ʽ�ζ���δ�ô�����Һ��ϴ | |
| B�� | ��ʽ�ζ���δ�ô�װ��Һ��ϴ | |
| C�� | ��ƿδ�ô�װ��Һ��ϴ | |
| D�� | �ڵζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ |
| ʱ�� | c��CO��/mol•L-1 | c��H2��/mol•L-1 | c��CH3OH��/mol•L-1 |
| ��ʼ | 1 | 3 | 0 |
| ��2min | 0.8 | 2.6 | 0.2 |
| ��4min | 0.4 | 1.8 | 0.6 |
| ��6min | 0.4 | 1.8 | 0.6 |
| A�� | ��4 min����6 min�û�ѧ��Ӧ����ƽ��״̬ | |
| B�� | ��2 minʱ�����ֻ�ı�ijһ��������ı���������ܼ�����H2 | |
| C�� | ��2 minʱ�����ֻ�ı�ijһ��������ı������������ʹ�ô��� | |
| D�� | ��6 minʱ�������������䣬��������¶ȣ�����Ӧ�������� |
 ����������һ��ʱ��ͼ�����߱�ʾ��Ӧ2NO��g��+O2��g��?2NO2��g����H��0��ƽ��ʱNO ��ת�������¶ȵĹ�ϵ��ͼ�ϱ���A��B��C��D��E�㣬�����й�˵����ȷ���ǣ�������
����������һ��ʱ��ͼ�����߱�ʾ��Ӧ2NO��g��+O2��g��?2NO2��g����H��0��ƽ��ʱNO ��ת�������¶ȵĹ�ϵ��ͼ�ϱ���A��B��C��D��E�㣬�����й�˵����ȷ���ǣ�������| A�� | A�㷴Ӧδ�ﵽƽ��״̬���ҷ�Ӧ�����ƶ� | |
| B�� | C��NOת������ͣ��һ�ѧ��Ӧ������С | |
| C�� | B��D�����������Ӧ�¶��µĻ�ѧƽ��״̬���Ҧ�B��NO������D��NO�� | |
| D�� | E�㷴Ӧδ��ƽ�⣬��Ӧ�����ƶ���������ѹǿ���� |
 ij��ѧѧϰС����о������ǣ�̽���ⶨ���ᾧ�壨H2C2O4•xH2O����x��ֵ������ͬѧͨ���������ϲ�Ѱ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���
ij��ѧѧϰС����о������ǣ�̽���ⶨ���ᾧ�壨H2C2O4•xH2O����x��ֵ������ͬѧͨ���������ϲ�Ѱ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���