��Ŀ����
14�� 1-����鳣�����л���Ӧ���ܼ���ʵ�����Ʊ�1-����飨CH3CH2CH2Br���ķ�Ӧ����Ҫʵ��װ�����£�
1-����鳣�����л���Ӧ���ܼ���ʵ�����Ʊ�1-����飨CH3CH2CH2Br���ķ�Ӧ����Ҫʵ��װ�����£���֪��
��CH3CH2CH2OH+HBr$\stackrel{��}{��}$CH3CH2CH2Br+H2O
��.2CH3CH2CH2OH$��_{140��}^{Ũ����}$��CH3CH2CH2��2O�������ѣ�+H2O
�����õ�������������£�
| ��Է� ������ | �ܶ� /g•mL-1 | �е�/�� | ˮ�� �ܽ��� | |
| ������ | 60 | 0.896 | 97.1 | �� |
| ������ | 102 | 0.74 | 90 | �������� |
| 1-����� | 123 | 1.36 | 71 | ���� |
����A�м���50g��������һ������Ũ���ᡢ�廯��һ����ȣ����뼸����ʯ�����ڱ���69��72���������2Сʱ���������ռ�68��90�����Һ ����̼������Һϴ�����ԣ���Һ�����������ռ�68��76�����Һ���õ�����1-�����66g����ش�
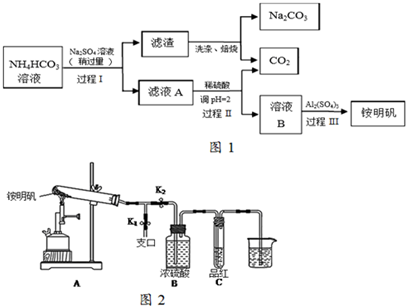
��1��Bװ�������ǣ����Σ������ܣ���ʯ�������Ƿ�ֹҺ�屩�У�
��2������Aǰ�����ȴ�b���a����b��������B��ͨ��ˮ��
��3������ܵ�Ŀ����Ҫ��ϴȥŨ���ᣬ��ҡ���ã��ֲ���Ӧ�ӷ�Һ©�����£���ϡ����¡����ڷ������
��4����ʵ�����õ���1-����������C������ȷ����ţ���
A.41%������B.50%������C.64%������D.70%
���� ��1��װ��ͼ������������BΪ�����ܣ���A�м��뼸����ʯ���������Ƿ�ֹҺ�屩�У�
��2������ˮ��ˮ����������������෴����������Ч���ã����¿ڽ����Ͽڳ���
��3���������̼������Һϴ�����ԣ���Ҫ�dz�ȥ���ᣬ1-�������ܶȱ�ˮ��
��4�����ݻ�ѧ��Ӧ����ʽ�Ͳ��ʵļ���ɵã�
��� �⣺��1��Bװ�þ�����������������������ΪҺ�壬���������������ܣ���ʯ������ͨ����ʯ�����Ƭ����϶����������ʹ��Ϊ���ݸ��������������Ƿ�ֹҺ�屩�У�
�ʴ�Ϊ�������Σ������ܣ���ֹҺ�屩�У�
��2������ˮ��ˮ����������������෴���¿ڽ����Ͽڳ������Լ���Aǰ�����ȴӡ�b������B��ͨ��ˮ���˷�ˮ���������������ܣ�����������������Ч���ã�
�ʴ�Ϊ��b��
��3���������̼������Һϴ�����ԣ���Ҫ�dz�ȥ���ᣬ1-�������ܶȱ�ˮ�����²㣬Ӧ�ӷ�Һ©�����¿�������
�ʴ�Ϊ��Ũ����£���
��4�����ݻ�ѧ��Ӧ����ʽCH3CH2CH2OH+HBr$\stackrel{��}{��}$CH3CH2CH2Br+H2O�ͱ������ݿ�֪��60g��������������123 g 1-����飬��ô50 g������������ȡ1-�����102.5 g����ʵ�ʵõ�66 g�����Բ���Ϊ$\frac{66}{102.5}$��100%��64%��
�ʴ�Ϊ��C��
���� ���⿼����1-������Ʊ���ע�����֪ʶ����Ӧԭ�����������պ�ʵ���������������Ӧ�ã���Ŀ�Ѷ��еȣ�

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д�| A�� | ��ʽ�ζ���ֻ��ˮϴ����δ�ô���Һ��ϴ | |
| B�� | ��ƿ�в���������ˮ | |
| C�� | ��ʽ�ζ��ܵζ�ǰ���촦�����ݣ��ζ���������ʧ | |
| D�� | �ζ�ǰ���ӿ̶ȶ������ζ����ӿ̶ȶ��� |
 ����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����| ����a | ��̿���ڸ��������»�ԭCuO |
| ����b | �������ǻ�ԭ���Ƶ�Cu��OH��2�Ʊ�Cu2O�� |
| ����c | ��ⷨ����ӦΪ2Cu+H2O$\frac{\underline{\;���\;}}{\;}$Cu2O+H2���� |
| ����d | ���£�N2H4����ԭ���Ƶ�Cu��OH��2 |
��C��s��+$\frac{1}{2}$O2��g��=CO��g������H=-110.5kJ•mol-1
��Cu��s��+$\frac{1}{2}$O2��g��=CuO��s������H=-157kJ•mol-1
��a�������Ȼ�ѧ����ʽ�ǣ�C��s��+2CuO��s��=Cu2O��s��+CO��g����H=+34.5kJ•mol-1��
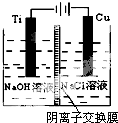
��2������c�������ӽ���Ĥ���Ƶ��Һ��OH-��Ũ�ȶ��Ʊ�����Cu2O��װ����ͼ��ʾ��
�����ӽ���ĤΪ�����ӽ���Ĥ������������������õ�ص�������ӦʽΪ2Cu-2e-+2OH-=Cu2O+H2O���Ѽ�������pHֵ�����������С�����䡱����
��3������dΪ������������Һ̬�£�N2H4����ԭ����Cu��OH��2���Ʊ�����Cu2O��ͬʱ�ų�N2�����Ʒ��Ļ�ѧ����ʽΪ4Cu��OH��2+N2H4$\frac{\underline{\;\;��\;\;}}{\;}$2Cu2O+N2��+6H2O��
��4������ͬ���ܱ������У������Ϸ����Ƶõ�����Cu2O�ֱ���д��ֽ�ˮ��ʵ�飺2H2O��g��$?_{Cu_{2}O}^{����}$2H2��g��+O2��g����H��0��ˮ������Ũ����ʱ��t�仯���±���ʾ��
| ��� | 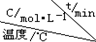 | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
��ʵ���ǰ20min��ƽ����Ӧ���� v��O2��=3.5��10-5mol/��L•min��
�۴����Ĵ�Ч�ʣ�ʵ��٣�ʵ��ڣ��������������
| A�� | �ñ�FeCl3��Һ�ζ�KI��Һ��ѡ��KSCN��Һ | |
| B�� | ��I2��Һ�ζ�Na2SO3��Һ��������ָʾ�� | |
| C�� | ��AgNO3��Һ�ζ�NaCl��Һ��Na2CrO4��ָʾ�� | |
| D�� | ��H2O2��Һ�ζ�KI��Һ��������ָʾ�� |
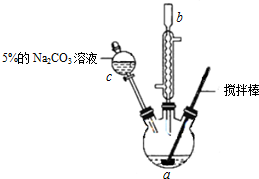
��ʵ�鲽�����£�
��������ƿ�����μ��룺10.0mL��ϩ�ᣨCH2=CHCOOH����������Ũ���ᡢ10.0mL��ˮ�״���
2����ʯ����ͼʾװ�����Ӻ������ܣ��ý�������裬ˮԡ���ȣ�
�ڳ�ַ�Ӧ����ȴ������Һ�м���5% Na2CO3��Һϴ�����ԣ�
�۷�Һ��ȡ�ϲ���״Һ�壬������ˮNa2SO4����������ռ�70�桫90����֣�
�����õ�����Ϣ��
| �ܶ� | �е� | �ܽ��� | ||
| ��ϩ�� | 1.05g/cm3 | 141�� | ��ˮ���ܣ��������л��ܼ� | �ж� |
| �״� | 0.79g/cm3 | 64.7�� | ��ˮ���ܣ��������л��ܼ� | �ӷ����ж� |
| ��ϩ����� | 0.95g/cm3 | 80.5�� | ������ˮ���������л��ܼ� | �ӷ� |
��1������c�������Ƿ�Һ©����
��2�����Һ��5% Na2CO3��Һϴ�ӵ������dz�ȥ��ϩ�ᡢŨ������������ʣ�
��3����ʵ����Ӧ���õİ�ȫ������ʩ��ͨ�����ʵ�飮��1�����ɣ�
��Ϊ�ⶨ������Ӧ�б�ϩ������IJ��ʣ��������ʵ�飺
�ٽ���״�����ᴿ��ƽ���ֳ�5�ݣ�ȡ��1��������ƿ�У�����2.5mol/L��KOH��Һ10.0mL������ʹ֮��ȫˮ�⣮
���÷�̪��ָʾ��������ȴ�����Һ�еμ�0.5mol/L��HCl��Һ���к�����KOH���ε��յ�ʱ����������20.00mL��
��4����д�����������з����Ļ�ѧ��Ӧ����ʽCH2=CHCOOCH3+KOH$\stackrel{��}{��}$CH2=CHCOOK+CH3OH��
��5�����㱾��������Ӧ��ϩ������IJ��ʣ�
 ij��ѧѧϰС���ͬѧ ����������ԭ��Ӧ��MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O���ɲ��õζ��ķ����ⶨFeSO4������������ʵ�鲽�����£�
ij��ѧѧϰС���ͬѧ ����������ԭ��Ӧ��MnO4-+5Fe2++8H+�TMn2++5Fe3++4H2O���ɲ��õζ��ķ����ⶨFeSO4������������ʵ�鲽�����£�