��Ŀ����
19����֪K2Cr2O7��Һ�д�������ƽ�⣺Cr2O72-����ɫ��+H2O?2H++2CrO42-����ɫ��������2mL 0.1mol•L-1 K2Cr2O7��Һ�е���3��6mol•L-1 NaOH��Һ����Һ�ɳ�ɫ��Ϊ��ɫ����������Һ���ٵ���5��ŨH2SO4����Һ�ɻ�ɫ��Ϊ��ɫ��
�����ữK2Cr2O7��Һ�е�������Na2SO3��Һ����Һ�ɳ�ɫ��Ϊ��ɫ��
Cr2O72-+8H++3SO32-�T2Cr3+����ɫ��+3SO42-+4H2O�����з�����ȷ���ǣ�������
| A�� | CrO42-��S2-��������Һ�пɴ������� | |
| B�� | ʵ���˵�������ԣ�Cr2O72-��SO42- | |
| C�� | ϡ��K2Cr2O7��Һʱ����Һ�и�����Ũ�Ⱦ���С | |
| D�� | ʵ��ٺ͢ھ���֤��K2Cr2O7��Һ�д�������ƽ�� |
���� A��CrO42-���������ԣ�������Һ�����������ӣ�
B���������������Դ���������������жϣ�
C��ϡ����Һ��ƽ��״̬������Ũ�ȼ�С��������Ũ�ȼ�С����Һ�д������ӻ�������������Ũ������
D����Һ�ɳ�ɫ��Ϊ��ɫ����Һ�ɻ�ɫ��Ϊ��ɫ��˵����������ƽ���ƶ���
��� �⣺A��CrO42-���������ԣ�������Һ�����������ӣ�CrO42-��S2-��������Һ�в����Դ������棬��A����
B����Ӧ��Cr2O72-+8H++3SO32-�T2Cr3+����ɫ��+3SO42-+4H2O���������������Դ��������������ʵ�����˵�������ԣ�Cr2O72-��SO42-����B��ȷ��
C��ϡ��K2Cr2O7��Һʱ��ƽ��״̬������Ũ�ȼ�С��������Ũ�ȼ�С����ˮ�����ӻ������֪������������Ũ������C����
D����������������Һ����Һ�ɳ�ɫ��Ϊ��ɫ��˵��ƽ��������У�����������Һ�ɻ�ɫ��Ϊ��ɫ��˵��ƽ��������У�˵����������ƽ���ƶ���ʵ�����֤��K2Cr2O7��Һ�д�������ƽ�⣬ʵ��ڲ���֤��K2Cr2O7��Һ�д�������ƽ�⣬��D����
��ѡB��
���� ���⿼��������ˮ��ķ���Ӧ�ã�������ԭ��Ӧ�Ĺ��ɷ�����ƽ���ƶ�ԭ��������Ӧ�ã����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д� ��������ϵ�д�
��������ϵ�д� 25��ʱ��Fe��OH��3��s����Cu��OH��2��s���ֱ�����Һ�дﵽ�����ܽ�ƽ��ı���ҺpH������������Ũ�ȵı仯��ͼ��ʾ����ͼ�����������жϴ�����ǣ�������
25��ʱ��Fe��OH��3��s����Cu��OH��2��s���ֱ�����Һ�дﵽ�����ܽ�ƽ��ı���ҺpH������������Ũ�ȵı仯��ͼ��ʾ����ͼ�����������жϴ�����ǣ�������| A�� | pHΪ5ʱ���ɳ�ȥCuCl2��Һ�е�����Fe3+ | |
| B�� | �¶�����ʱFe��OH��3�ܽ��ٶȼӿ� | |
| C�� | 25����Ksp[Cu��OH��2]=1.0��10-20 | |
| D�� | Fe��OH��3��s�����ܽ����pH�������С |

��1����ʵ�����У�����98%��Ũ���ᣨ�ܶ�Ϊ1.84g•mL-1������500mL1.0mol•L-1�����ᣬ��Ҫ����������Ͳ���ձ����������⣬���н�ͷ�ιܡ�500mL����ƿ��
��2����С��ͬѧ�����ͼװ��ģ������ڹ��������б��գ�����֤�����к���Ԫ�أ�

��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����Ϊ���Ʒ�Ӧ�����ڼ��Ҳ�����ƽ����������ȡ�IJ����������Ǵ�Һ©���Ͽڻ��������Ʒ�Һ©��������ʹˮ������ε��£�B��Ӧ����ʢ�м�ʯ�ҵĸ���ܣ���U�ιܣ���Ũ�����ϴ��ƿ����д�Լ����������ƣ���
��Eװ���м���Ʒ����Һ��Ŀ���Ǽ�������a�е�SO2����Fװ���г��ְ�ɫ����ʱ����Ӧ���ӷ���ʽΪ2SO2+O2+2H2O+2Ba2+=2BaSO4��+4H+��
��3�����в����У������ڲ�����н��еIJ�������ad�������и�������ţ���

��4��Ϊ�ⶨ��Ʒ���̷���������������ȡ30.000g��Ʒ����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000mol•L-1����KMnO4��Һ���еζ�����ӦΪ��10FeSO4+8H2SO4+2KMnO4�T2MnSO4+5Fe2��SO4��3+K2SO4+8H2O��ʵ�������������±���ʾ��
| ����� | 1 | 2 | 3 | 4 |
| KMnO4��Һ���/mL | 20.90 | 20.02 | 19.98 | 20.00 |
a����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ b����ƿϴ����δ����
c���ζ��յ�ʱ���Ӷ��� d���ζ��յ�ʱ���Ӷ���
�ڸ��ݱ������ݣ��������ò�Ʒ���̷�����������Ϊ92.7%��

| A�� | �ȼҵ�У�X�缫�Ϸ�Ӧʽ��4OH--4e-�T2H2O+O2�� | |
| B�� | ��⾫��ͭʱ��Z��Һ�е�Cu2+Ũ�ȱ�С | |
| C�� | ��ȡ����þʱ��Z�����ڵ�����þ | |
| D�� | ����Ƭ�϶�ͭʱ��Y�Ǵ�ͭ |
| A�� | �ۢܢݢޢߢ� | B�� | �ܢݢ� | C�� | �ܢݢߢ� | D�� | �ۢܢݢ� |
| A�� | ȡa�˻�����ּ������������䣬����b�� | |
| B�� | ȡa�˻����������ϡ�����ַ�Ӧ�����ȡ����ɡ��������ۻ�����ȴ��b�˹��� | |
| C�� | ȡa�˻����������ϡ�����ַ�Ӧ���ݳ������ü�ʯ����ȫ���գ�����b�� | |
| D�� | ȡa�˻����������Ba��OH��2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b�˹��� |
 ��ͨ��״���£�AΪ��̬���ʣ�������ͼת����ϵ���ش�
��ͨ��״���£�AΪ��̬���ʣ�������ͼת����ϵ���ش�

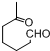 +NaOH+2Cu��OH��2$\stackrel{��}{��}$
+NaOH+2Cu��OH��2$\stackrel{��}{��}$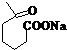 +Cu2O��+3H2O��
+Cu2O��+3H2O�� ����D��ૣ�
����D��ૣ� ��Ҳ���Է�����Diels-Alder��Ӧ�����û�ѧ��Ӧ����ʽΪ
��Ҳ���Է�����Diels-Alder��Ӧ�����û�ѧ��Ӧ����ʽΪ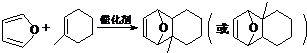 ��
��