��Ŀ����
9��ij��ѧС���������������������װ�ã���ͼ1�����Ի������Ʊ�����ϩ��֪��


��1���Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���l mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������Ƿ����У�����B���˵�������е�������������
���Թ�C���ڱ�ˮԡ�е�Ŀ���Ƿ�ֹ����ϩ�ӷ���
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȣ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ���ϲ�
�㣨��ϡ����¡�������Һ����c �������ţ�ϴ�ӣ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ��ͼ2װ��������ȴˮ��g���f����g�����ڽ��룮
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��83�����ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����c
a������ʱ��70�濪ʼ�ռ���Ʒ
b��������ʵ����������
c���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������bc��
a�������Ը��������Һ b���ý����� c���ⶨ�е� d����FeCl3��Һ
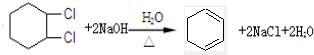
��4�����û���ϩ��������Ӧ�ϳ�1��3-������ϩ ��
 ������д����������Ӧ��Ӧ�Ļ�ѧ����
������д����������Ӧ��Ӧ�Ļ�ѧ����ʽ
 ��
�� ��
��
���� ������ݴ�����ȥ��Ӧԭ�������û������Ʊ�����ϩ��ʵ��������̣��漰�Ʊ��������ᴿ��ʵ����ۺ����ۣ��Ʊ������з�Ӧ���Һ����ʱΪ�˷�����Ҫ�������Ƭ�������ռ���Ҫ����ʹ֮Һ�������ӷ����ֲ�Ʒ����ʱ�漰����Һ��������ַ�����������̽���˲�Ʒ���ȷ�����
��1���ٸ�������ϩʵ���֪ʶ������װ��A�����Ƭ�������Ƿ�ֹ���У��������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ������
�ڱ�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ����
��2���ٻ���ϩ�������Ȼ�����Һ�����ܶȱ�ˮС���ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ��������ᴿ����ʱ��c��Na2CO3��Һ��ϴ�ӿɳ�ȥ�
������ʱΪ����������Ч������ȴˮ���¿ڣ�g�����룻
�۸��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棻
a������ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�����
b����ȡ�Ļ���ϩ���ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�����
c���ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�����
��3�����ݻ����û�й̶��ķе㣬���������й̶��ķе㣬�ݴ˿��жϲ�Ʒ�Ĵ��ȣ������Ը��ݻ������뻷��ϩ�Ľṹ����������
��4���û���ϩ��������Ӧ�ϳ�1��3-������ϩ���ɽ��ϩ���ļӳɺ�±��������ȥ��Ӧԭ����������
��� �⣺��1���ٸ�������ϩʵ���֪ʶ������װ��A�����Ƭ�������Ƿ�ֹ���У��������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ�������ʴ�Ϊ����ֹ���У�������
�ڱ�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ�����ʴ�Ϊ����ֹ����ϩ�ӷ���
��2���ٻ���ϩ�����࣬�������Ȼ�����Һ�����ܶȱ�ˮС�������á��ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ����������룺�Ʊ����������ᴿ����ʱ��c������Na2CO3��Һ��ϴ�ӿɳ�ȥ��ʴ�Ϊ���ϲ㣻c��
������װ��Ҫ�������ܣ�Ϊ����������Ч������ȴˮ�ķ���Ӧ�ú������������෴����ȴˮ���¿ڣ�g�����룬�ʴ�Ϊ��g��
�۸��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棬���ռ���ƷӦ�����¶���83�����ң�
a������ʱ��70�濪ʼ�ռ���Ʒ����ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�������a����
b��������ʵ���������ˣ���ȡ�Ļ���ϩ�����ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�������b����
c�����ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�������c��ȷ����ѡc��
�ʴ�Ϊ��83�棻c��
��3�������Ʒ�뾫Ʒ�ɼ�������ƣ��۲��Ƿ�������������������壬���Ǿ�Ʒ��������ݻ����û�й̶��ķе㣬���������й̶��ķе㣬ͨ���ⶨ����ϩ��Ʒ�ͻ���ϩ��Ʒ�ķе㣬Ҳ���жϲ�Ʒ�Ĵ��ȣ��ʴ�Ϊ��bc��
��4������ϩ�������������ӳɷ�Ӧ�������������ƵĻ����Һ��ϼ��ȣ�����±��������ȥ��Ӧ���ɵ�1��3-������ϩ��������Ӧ�Ļ�ѧ����ʽΪ
����ϩ��ˮ�����ӳɷ�Ӧ���ɻ���������ѧ����ʽΪ ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
���� ���⿼�����Ի������Ʊ�����ϩ��ʵ�鷽�����ۺϿ��������ʵķ��뷽���������������ķ����ȣ��Ѷ����У�����ѧ�����ʵ�������������

 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�| A�� | KCl��Һ��Na2CO3��Һ��Ӧ��2K++CO32-�TK2CO3 | |
| B�� | ����þ�����ᷴӦ��MgO+2H+�TMg2++H2O | |
| C�� | ̼�����������ᷴӦ��CO32-+2H+�TCO2��+H2O | |
| D�� | ̼�������Ȼ��Ʒ�Ӧ��Na2CO3+Ca2+�TCaCO3��+2Na+ |
��1�����³�ѹ�£�������̼�����ѡ�������ɶ����ѻ�һ����̼��
��CO2��g��+3H2��g��?CH3OH��l��+H2O��l����H=-55.7kJ/mol
��2CH3OH��l��?CH3OCH3��g��+H2O ��l����H=-23.4kJ/mol
��CO2��g������ת��ΪCH3OCH3��g����H2O ��l�����Ȼ�ѧ����ʽ�ǣ�2CO2��g��+6H2��g��=CH3OCH3��g��+3 H2O��l����H=-134.8kJ/mol��
��2��CO2�ϳ����صķ�ӦΪ��CO2��g��+2NH3��g��?CO��NH2��2��l��+H2O ��g����H��0���÷�Ӧ��ƽ�ⳣ������ʽΪ$\frac{c��{H}_{2}O��}{c��C{O}_{2}����{c}^{2}��N{H}_{3}��}$��
��3��ʵ����ģ����CO��H2��Ӧ���Ƽ״���CO��g��+2H2��g��?CH3OH��g����H��0����250���£���һ������CO��H2Ͷ��10L���ܱ������У������ʵ����ʵ���Ũ�ȣ�mol•L-1���仯���±���ʾ����ǰ6minû�иı�������
| 2min | 4min | 6min | 8min | �� | |
| CO | 0.07 | 0.06 | 0.06 | 0.05 | �� |
| H2 | x | 0.12 | 0.12 | 0.2 | �� |
| CH3OH | 0.03 | 0.04 | 0.04 | 0.05 | �� |
��250��ʱ�÷�Ӧ��ƽ�ⳣ��KֵΪ��$\frac{0.04}{0.06��0.1{2}^{2}}$mol-2��L2�����ػ���
����6min��8minֻ�ı���ijһ���������ı�������Ǽ���1mol������
�ܵ�8minʱ���÷�Ӧ�Dz��Ǵﵽƽ��״̬���ǣ�����ǡ����ǡ���
�ݸúϳɷ�Ӧ���¶�һ�������240��270�棬ѡ����¶ȵ�ԭ���ǣ����¶��µĴ������Ըߣ����¶ȵͣ���Ӧ����������λʱ���ڵIJ����ͣ�������ӦΪ���ȷ�Ӧ���¶ȹ��ߣ�ת���ʽ��ͣ�
| �� | �� | �� | �� | ||
| ��ʼ���ʵ��� | n��SO2��/mol | 0.40 | 0 | 0.80 | 0.02 |
| n��O2��/mol | 0.24 | 0 | 0.48 | 0.04 | |
| n��SO3��/mol | 0 | 0.40 | 0 | 0.40 | |
| ����Ӧ���ƽ��ת����% | 80 | a1 | a2 | a3 | |
| A�� | ���¶��£�ƽ�ⳣ����ֵΪ400 | B�� | ƽ��ʱ������c��SO3���Ǽ��е�2�� | ||
| C�� | ƽ��ʱ��a3��a1 | D�� | ����SO3��ƽ��ת����Ϊa1=20% |
| AX | BX | AY | BY |
| pH=7��c��X-��=1mol/L | pH=4 | pH=6 |
��2������Ũ�ȵ�HX��HYϡ����ͬ�ı�������Һ��pH�仯���ȣ�HX��HY���������������pH��AOH��BOH��Һ��ϡ��ʱpH���½�2�����ˮ����AOH��BOH�������������������=������
��3����BX��Һ�У�c��B+��+c��H+��-c��OH-��=1mol/L��
��AY��Һ�У�c��A+��-c��Y-��=c��HY������һ���ʾ��=c��OH-��-c��H+�����������ʾ����
��4��A2Z��ˮ�ⷽ��ʽ��Z2-+H2O?HZ-+OH-��HZ-+H2O?H2Z+OH-��
| A�� | ��������SO2��Ʒ����Һ | |
| B�� | ��HClͨ��NaAlO2��Һ�� | |
| C�� | ��Fe��NO3��2��Һ�еμ�ϡ���� | |
| D�� | ��̼��������Һ�еμ�����������Һ |
 ������ӵ�����������2�֣��Ƚ�����ͬ����ǽ���Ԫ���γɵĵ��ʵ������ԣ��û�ѧ����ʽ��ʾ��2H2S+O2=2S��+2H2O
������ӵ�����������2�֣��Ƚ�����ͬ����ǽ���Ԫ���γɵĵ��ʵ������ԣ��û�ѧ����ʽ��ʾ��2H2S+O2=2S��+2H2O ����Ҫ��Br-��ʽ�����ں�ˮ�У���ˮ�������ԣ���ҵ���Ʊ���Br2�IJ�������Ϊ��
����Ҫ��Br-��ʽ�����ں�ˮ�У���ˮ�������ԣ���ҵ���Ʊ���Br2�IJ�������Ϊ��