��Ŀ����
��ˮ�����ظ����ƣ�Na2Cr2O7.2H2O����[��֪M��Na2Cr2O7.2H2O��=298g/mol]�׳ƺ췯�ƣ��ڹ�ҵ�����й㷺��;���ҹ�Ŀǰ��Ҫ���Ը�������Ҫ�ɷ�ΪFeO.Cr2O3��������A1203��MgO��Si02�����ʣ�Ϊ��Ҫԭ������������Ҫ����������ͼ��
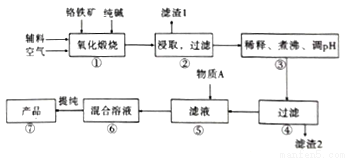
��֪��Ϣ��
��.���١����漰����Ҫ��Ӧ�ǣ�4FeO��Cr2O3��8Na2CO3��7O2=8Na2CrO4��2Fe2O3��8CO2
��.����������������������ʽ��ȫ����ʱ��Һ��PH���£�
������ | Al��OH��3 | Fe��OH��3 | Mg��OH��2 | Cr��OH��3 |
��ȫ����ʱ��ҺPH | 4.7 | 3.7 | 11.2 | 5.6 |
��1��������A12O3��SiO2�봿�Ӧת��Ϊ�������Σ�д��A12O3���Ӧ�Ļ�ѧ����ʽΪ��______________������1����Ҫ�ɷ�Ϊ__________________��
��2�������ۡ���ͨ������PH��ȥ���ʣ�����PH��___________��
��3�������ݡ�������Һ�м�������A���õ�Na2Cr2O3��Na2SO4�Ļ����Һ������AΪ__________������ԭ��______________________________���������ӷ���ʽ�����ּ���˵����
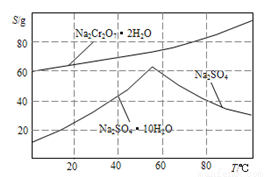
��4������ͼ��Na2Cr2O3.2H2O��Na2SO4���ܽ�����ߡ��ᴿ��ʵ���������Na2Cr2O3��Na2SO4�Ļ����Һ______________________���õ�Na2SO4����ͽϴ�����Na2Cr2O3��Һ��
��5����ʵ���Ҳⶨ���ò�Ʒ�к췯�ƵĴ��ȣ���ȡ��Ʒ3.2g�����250mol��Һ��ȡ��25.00mL����ƿ�У�����10mL2mol/LH2SO4�������⻯�أ����ڰ���5min��д�����ӷ���ʽ��________________��Ȼ���ˮ100mL������3mL����ָʾ������0.1000mol/LNa2S2O3����Һ�ζ�I2+2S2O32-=2I-+S4O62-���ﵽ�ζ��յ�ʱ������ȥNa2S2O3����Һ60mL�����ò�Ʒ�Ĵ�����_________���������С�����1λ����
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�| A�� | Na2CO3��ĩ��NaHCO3��������ֱ�������������ټ��� | |
| B�� | NaHCO3��Һ��Na2CO3��������Һ��ͨ�������CO2���� | |
| C�� | CO2��HCl��������������ͨ��Na2CO3��Һ��Ũ������ | |
| D�� | H2��HCl����������ͨ���ʯ���� |
| ���������� �͵缫��Ӧʽ | ���������� �͵缫��Ӧʽ | ����ܷ�Ӧʽ | ��ҺŨ�ȱ仯 | ��ҺpH�ı仯 | |
| HCl | 2Cl--2e-=Cl2�� | 2H++2e-=H2�� | 2Cl-+2H+$\frac{\underline{\;ͨ��\;}}{\;}$Cl2��+H2�� | ��С | ���� |
| CuBr2 | 2Br--2e-=Br2 | Cu2++2e-=Cu | 2Br-+Cu2+$\frac{\underline{\;ͨ��\;}}{\;}$Br2+Cu | ��С | ���� |
| KCl | 2Cl--2e-=Cl2�� | 2H++2e-=H2�� | 2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$Cl2��+H2��+2OH- | ��С | ���� |
| CuSO4 | 4OH--4e-=O2��+2H2O | Cu2++2e-=Cu | 2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+ | ��С | ���� |
| NaOH | 4OH--4e-=O2��+2H2O | 2H++2e-=H2�� | 2H2O$\frac{\underline{\;ͨ��\;}}{\;}$O2��+2H2�� | ���� | ���� |
| H2SO4 | 4OH--4e-=O2��+2H2O | 2H++2e-=H2�� | 2H2O$\frac{\underline{\;ͨ��\;}}{\;}$O2��+2H2�� | ���� | ��С |
| KNO3 | 4OH--4e-=O2��+2H2O | 2H++2e-=H2�� | 2H2O$\frac{\underline{\;ͨ��\;}}{\;}$O2��+2H2�� | ���� | ���� |
| Na2SO4 | 4OH--4e-=O2��+2H2O | 2H++2e-=H2�� | 2H2O$\frac{\underline{\;ͨ��\;}}{\;}$O2��+2H2�� | ���� | ���� |


 ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺