��Ŀ����
15�����屽��ϩ���ϩ�Ĺ�����E��һ�ָ߷�����ȼ�������еͶ������ȶ��Ժõ��ŵ㣮����A�ϳ�E��·����ͼ��ʾ��
�ش��������⣺
��1��A�������ұ����ܵķ�Ӧ����Ũ���ᡢ���ȣ�
��2��������E�Ľṹ��ʽ
 ��
�� ��
����3����Ӧ�ٵĻ�ѧ����ʽ
 +Br2$\stackrel{��}{��}$
+Br2$\stackrel{��}{��}$ +HBr����Ӧ�۵Ļ�ѧ����ʽ
+HBr����Ӧ�۵Ļ�ѧ����ʽ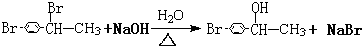 ��
����4����������������C��ͬ���칹��Ľṹ��ʽ
 ����дһ�֣���
����дһ�֣���i����ʹFeCl3��Һ����ɫ ii���˴Ź���������ʾ��3��������Ϊ6��2��1��
��5����ϩ�����۵õ�2��3-����-1-��ϩ��F��2��3-����-1-��ϩ��Ϊͬ���칹�壬������̼ԭ�Ӵ���ͬһƽ�棬д��F�Ľṹ��ʽ��CH3��2C=C��CH3��2��
��֪��

���ϳ�·�߳��õı�ʾ��ʽΪA$��_{��Ӧ����}^{��Ӧ�Լ�}$B��$��_{��Ӧ����}^{��Ӧ�Լ�}$Ŀ����
���� ���ݸ��� �Ľṹ��������и�����ת����ϵ��֪����AΪ
�Ľṹ��������и�����ת����ϵ��֪����AΪ ��A���巢��ȡ����Ӧ����BΪ
��A���巢��ȡ����Ӧ����BΪ ��B�ڹ������������巢��ȡ����Ӧ����
��B�ڹ������������巢��ȡ����Ӧ���� ��
�� ����ˮ������CΪ
����ˮ������CΪ ��C������ȥ��Ӧ��DΪ
��C������ȥ��Ӧ��DΪ ��D����ϩ�����Ӿ۷�Ӧ��EΪ
��D����ϩ�����Ӿ۷�Ӧ��EΪ ��
�� ���ݴ˴��⣮
���ݴ˴��⣮
��� �⣺���ݸ��� �Ľṹ��������и�����ת����ϵ��֪����AΪ
�Ľṹ��������и�����ת����ϵ��֪����AΪ ��A���巢��ȡ����Ӧ����BΪ
��A���巢��ȡ����Ӧ����BΪ ��B�ڹ������������巢��ȡ����Ӧ����
��B�ڹ������������巢��ȡ����Ӧ���� ��
�� ����ˮ������CΪ
����ˮ������CΪ ��C������ȥ��Ӧ��DΪ
��C������ȥ��Ӧ��DΪ ��D����ϩ�����Ӿ۷�Ӧ��EΪ
��D����ϩ�����Ӿ۷�Ӧ��EΪ ��
�� ��
��
��1��AΪ ��A�������ұ�����Ӧ��Ϊ������ȥ��Ӧ����Ӧ����ΪŨ���ᡢ���ȣ�
��A�������ұ�����Ӧ��Ϊ������ȥ��Ӧ����Ӧ����ΪŨ���ᡢ���ȣ�
�ʴ�Ϊ���ұ���Ũ���ᡢ���ȣ�
��2����������ķ�����֪��������E�Ľṹ��ʽ Ϊ ��
�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
��3����Ӧ��Ϊ�ұ����巢�������ϵ�ȡ������Ӧ�Ļ�ѧ����ʽΪ +Br2$\stackrel{��}{��}$
+Br2$\stackrel{��}{��}$ +HBr����Ӧ��Ϊ±�����ļ���ˮ�⣬��Ӧ�Ļ�ѧ����ʽΪ��
+HBr����Ӧ��Ϊ±�����ļ���ˮ�⣬��Ӧ�Ļ�ѧ����ʽΪ��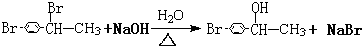 ��
��
�ʴ�Ϊ�� +Br2$\stackrel{��}{��}$
+Br2$\stackrel{��}{��}$ +HBr��
+HBr��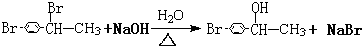 ��
��
��4��CΪ ����������i����ʹFeCl3��Һ����ɫ��˵���з��ǻ���ii���˴Ź���������ʾ��3��������Ϊ6��2��1������������������C��ͬ���칹��Ľṹ��ʽ Ϊ
����������i����ʹFeCl3��Һ����ɫ��˵���з��ǻ���ii���˴Ź���������ʾ��3��������Ϊ6��2��1������������������C��ͬ���칹��Ľṹ��ʽ Ϊ ��
��
�ʴ�Ϊ�� ��
��
��5����ϩ�����۵õ�2��3-����-1-��ϩ��F��2��3-����-1-��ϩ��Ϊͬ���칹�壬������̼ԭ�Ӵ���ͬһƽ�棬��F���ĸ�������̼̼˫������̼ԭ�ӣ���ṹ��ʽΪ��CH3��2C=C��CH3��2��
�ʴ�Ϊ����CH3��2C=C��CH3��2��
���� ���⿼���л���������ʺ��ƶϣ����ؿ���ѧ��֪ʶ�ۺ�Ӧ����������Ҫѧ���Գ����л�������ż��������������ղ�������ã��Ѷ��еȣ�

| ѡ�� | �� �� | Ҫ �� |
| A | K+��AlO2-��Cl-��MnO4- | c��K+����c��Cl-�� |
| B | Fe3+��NO3-��I-��HCO3- | ��������������������� |
| C | NH4+��Al3+��SO42-��CH2COOH | ��μ���NaOH��Һ������������� |
| D | Na+��Cu2��Cl-��SO42- | ��μӰ�ˮ���г����������������ʧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���������Һ���Ҷ��ᣨ���ᣩ��Ӧ��2MnO4-+5C2O42-+16H+��2Mn2++10CO2��+8H2O | |
| B�� | ������̼������Һ�� +CO32-�� +CO32-�� +H2CO3 +H2CO3 | |
| C�� | ����������ʵ���Ũ�ȵ�NH4Fe��SO4��2��Ba��OH��2��ϣ�2Fe3++3SO42-+3Ba2++6OH-�T3BaSO4��+2Fe��OH��3�� | |
| D�� | ��ǿ������Һ�д���������Fe��OH��3��Ӧ����Na2FeO4��3ClO-+2Fe��OH��3�T2FeO42-+3Cl-+H2O+4H+ |


 $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$ +H2O����Ӧ��������ȥ��Ӧ��
+H2O����Ӧ��������ȥ��Ӧ�� Ϊ��ʼԭ���Ʊ�
Ϊ��ʼԭ���Ʊ� �ĺϳ�·�ߣ����Լ����ã����ϳ�·��ʾ�����£�H2C=CH2$\stackrel{HBr}{��}$CH3CH2Br$��_{��}^{NaOH��Һ}$CH3CH2OH��
�ĺϳ�·�ߣ����Լ����ã����ϳ�·��ʾ�����£�H2C=CH2$\stackrel{HBr}{��}$CH3CH2Br$��_{��}^{NaOH��Һ}$CH3CH2OH��
 ��
�� �Т١��ڡ���3��-OH��������ǿ������˳���ǣ��ۣ��٣��ڣ�
�Т١��ڡ���3��-OH��������ǿ������˳���ǣ��ۣ��٣��ڣ�



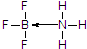 ����ע�����е���λ���������á�±�����ɺϳɺ�B��N����Ԫ�صĹ����մɣ�ͼ1Ϊ�þ���ľ����ṹ���ù����մɾ���Ļ�ѧʽΪBN��
����ע�����е���λ���������á�±�����ɺϳɺ�B��N����Ԫ�صĹ����մɣ�ͼ1Ϊ�þ���ľ����ṹ���ù����մɾ���Ļ�ѧʽΪBN�� ��1��Ŀǰ��ҵ�ϳɰ���ԭ����N2��g��+3H2��g��?2NH3��g����H=-93.0kJ•mol-1��
��1��Ŀǰ��ҵ�ϳɰ���ԭ����N2��g��+3H2��g��?2NH3��g����H=-93.0kJ•mol-1��