��Ŀ����
17��ijС����CoCl2•6H2O��NH4Cl��H2O2��Ũ��ˮΪԭ�ϣ��ڻ���̿���£��ϳ��˳Ȼ�ɫ����Cox��NH3��y ClZ��Ϊ�ⶨ����ɣ���������ʵ�飮���IJⶨ����ȷ��ȡwg��Ʒ��������ˮ�ܽ⣬ע����ͼ��ʾ������ƿ�У�Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������V1mLc1mol•L-1���������Һ���գ�����������ȡ�½���ƿ����c2mol•L-1 NaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����V2mLNaOH��Һ��
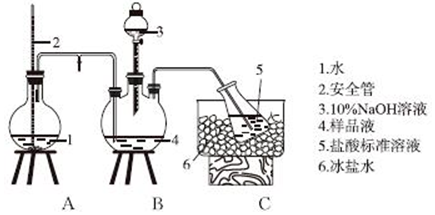
���IJⶨװ�ã���ʡ�Լ��Ⱥͼӳ�װ�ã�
���ȵIJⶨ����ȷ��ȡwg��Ʒ�������Һ����AgNO3����Һ�ζ�����֪��AgClΪ��ɫ����Ksp��AgCl��=1.8��10-10��Ag2CrO4Ϊש��ɫ������Ksp��Ag2CrO4��=1.2��10-12��Ag2SΪ��ɫ������Ksp��Ag2S��=6.3��10-50��
������Ʒ����Ϊwg����ȷ����Ʒ��CoԪ�ص�����������ȷ����Ʒ�Ļ�ѧ��ɣ�
�ش��������⣺
��1��ʵ��ǰ����ͼ��װ��װ�ú���μ���װ�õ������������Ӻ�װ�ú�����һ������ˮ�У�������סA�е���ƿ�������ܿ�������ð���������ֺ����γ�һ��ˮ���������������ã�
��2��ʢװ10%NaOH��Һ���������Ʒ�Һ©����
��3����Ʒ�а���������������ʽΪ$\frac{1{0}^{-3}��{c}_{1}{V}_{1}{-c}_{2}{V}_{2}��mol��17g/mol}{wg}$��
��4������������ҺӦװ����ɫ����ʽ�ζ����У����ζ���δ�ñ�Һ��ϴ����ⶨCl-����ƫ��
�����ƫ��ƫС����
��5���ڲⶨ�ȵĹ����У�Ӧѡ��K2S���K2CO3����K2S����Ϊָʾ�����жϴﵽ�ⶨ�յ�ʱ�IJ���������Ϊ�������һ�α���Һ�����ɺ�ɫ��������30s ����ԭ��
��6�����ﵽ�ζ��յ�ʱ����c��Ag+��=1.0��10-5 mol•L-1��$\frac{c��C{l}^{-}��}{c��Cr{O}_{4}^{2-}��}$=������$\frac{c��C{l}^{-}��}{c��{S}^{2-}��}$=��2.86��1034��������ѡָʾ��������գ�
���� ��1���跨ʹװ�������γ���ѹ���������Լ��ij����ֶΣ����������װ�������Ե�����������̼��ɽ��
��2���������ṹ��������֪ʢװ10%NaOH��Һ���������ƣ�
��3�����ݰ�����Ͱ�����Ӧ����֮��Ĺ�ϵʽ���㰱�����������ٸ�������������ʽ���㰱����������
��4��������Һֻ��ʢ������ʽ�ζ����У�����n��Cl-��=c��AgNO3��•V��AgNO3���������������������������Ӱ�죬�Դ��ж�Cl-��������
��5��K2SΪָʾ����Ag2SΪש��ɫ���ñ��������ζ�����Һ���ζ��յ�������ǵ������һ�α���Һ�����ɺ�ɫ��������30s ����ԭ��
��6���������ӻ�������c��Ag+��=1.0��10-5 mol•L-1����$\frac{c��C{l}^{-}��}{c��{S}^{2-}��}$��
��� �⣺��1��ʵ��ǰ����ͼ��װ��װ�ú�����ĩ������ˮ�У�������סA�е���ƿ�������ܿ�������ð���������ֺ����γ�һ��ˮ���������������ã�
�ʴ�Ϊ�������Ӻ�װ�ú�����һ������ˮ�У�������סA�е���ƿ�������ܿ�������ð���������ֺ����γ�һ��ˮ���������������ã�
��2���������ṹ��������֪ʢװ10%NaOH��Һ������Ϊ��Һ©����
�ʴ�Ϊ����Һ©����
��3���백����Ӧ��n��HCl��=10-3V1L��c1mol•L-1-c2mol•L-1 ��10-3V2L=10-3��c1V1-c2V2��mol�����ݰ�����HCl�Ĺ�ϵʽ֪��n��NH3��=n��HCl��=10-3��c1V1-c2V2��mol��������������=$\frac{1{0}^{-3}��{c}_{1}{V}_{1}{-c}_{2}{V}_{2}��mol��17g/mol}{wg}$��100%��
�ʴ�Ϊ��$\frac{1{0}^{-3}��{c}_{1}{V}_{1}{-c}_{2}{V}_{2}��mol��17g/mol}{wg}$��
��4��������Һֻ��ʢ������ʽ�ζ����У�����ʢ����Һ���������Ϊ��ʽ�ζ��ܣ�װ��Һ�ĵζ���δ�ñ�Һ��ϴ��Ũ�ȼ�С�����V��AgNO3��ƫ����n��Cl-��=c��AgNO3��•V��AgNO3����������֪Cl-����ƫ��
�ʴ�Ϊ����ʽ�ζ��ܣ�ƫ��
��5���ζ��յ�������ǵ������һ����������Һʱ����Һ�г��ֺ�ɫ��������������30s ����ԭ��
�ʴ�Ϊ���������һ�α���Һ�����ɺ�ɫ��������30s ����ԭ��
��6��c��Cl-��=$\frac{Ksp}{c��A{g}^{+}��}$=$\frac{1.8��1{0}^{-10}}{1.0��1{0}^{-5}}$=1.8��10-5��c��S2-��=$\frac{Ksp}{{c}^{2}��A{g}^{+}��}$=$\frac{6.3��1{0}^{-50}}{��1.0��1{0}^{-5}��^{2}}$=6.3��10-40��$\frac{c��C{l}^{-}��}{c��{S}^{2-}��}$=$\frac{1.8��1{0}^{-5}}{6.3��1{0}^{-40}}$=2.86��1034��
�ʴ�Ϊ��2.86��1034
���� ���⿼�������ʺ����IJⶨ���漰��������ܽ�ƽ�⡢������ԭ��Ӧ�����ʺ����IJⶨ��֪ʶ�㣬��ȷʵ��ԭ���ǽⱾ��ؼ���֪��ָʾ����ѡȡ���������װ�õ���������ʵ������ȡ�������Ҫ���裬Ӧ���������գ���Ŀ�Ѷ��еȣ�

 X��Y��ZΪԭ��������������Ķ���������Ԫ�أ�����Ԫ�����ڲ�ͬ���ڣ�����ת����ϵ�У�A��B��C��X��Y��Z��Ӧ��������̬���ʣ������Ϊ������������з�����ȷ���ǣ�������
X��Y��ZΪԭ��������������Ķ���������Ԫ�أ�����Ԫ�����ڲ�ͬ���ڣ�����ת����ϵ�У�A��B��C��X��Y��Z��Ӧ��������̬���ʣ������Ϊ������������з�����ȷ���ǣ�������| A�� | ���Ӱ뾶��Y��Z | B�� | Z�ĺ������Ϊǿ�� | ||
| C�� | ��Yͬ�����軯����D���ȶ� | D�� | F�����Ӽ����ۼ� |
 MnSO4�ڹ�ҵ������ҪӦ�ã������̿���Ҫ�ɷ�ΪMnO2��ˮ������Fe2O3��FeO��Al2O3������PbO�����ʣ������Ʊ�MnSO4����������£�
MnSO4�ڹ�ҵ������ҪӦ�ã������̿���Ҫ�ɷ�ΪMnO2��ˮ������Fe2O3��FeO��Al2O3������PbO�����ʣ������Ʊ�MnSO4����������£�I�� �����̿���ͨ��SO2���̡���������ǦԪ����������ʽ��������ý���Һ��pH��2��
II�� �����Һ�м�MnO2����ַ�Ӧ����ʯ���飬����ҺpH=4.7��
III���ټ��������������������ú���ˣ�
IV����Һ����Ũ������ȴ�ᾧ�����MnSO4���壮
�����ϡ������������γ��������������pH
| ���� | Fe2+ | Fe3+ | Al3+ | Mn2+ | Pb2+ |
| ��ʼ����ʱ��pH | 7.6 | 2.7 | 3.8 | 8.3 | 8.0 |
| ��ȫ����ʱ��pH | 9.7 | 3.7 | 4.7 | 9.8 | 8.8 |
��2��II �м���MnO2����ҪĿ���ǽ�Fe2+����ΪFe3+������Һ pH=4.7�����ɵij�����Ҫ����Fe��OH��3��Al��OH��3
������CaSO4��
��3��III�м�������������������Ҫ��ȥ��������Ca2+��Pb2+��
��4���ö��Ե缫���MnSO4��Һ�������Ƶø���MnO2��
�ٵ��MnSO4��Һ�����ӷ���ʽ��Mn2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$MnO2+H2��+2H+��
�ڸ���MnO2�����ڴ������ȩ���й����ı仯��ͼ��ʾ������X��HCO3-���ܷ�Ӧ�Ļ�ѧ����ʽ��HCHO+O2$\frac{\underline{\;MnO_{2}\;}}{\;}$CO2+H2O��
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2 054 | 1 535 | 1 462 |
| �е�/�� | 2 467 | 2 980 | 2 750 | - |
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���NaOH��Һ����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��3��ʵ�����ܽ��������������Լ��������˵��Լ���B������ţ���
A��Ũ���� B��ϡ���ᡡ������C��ϡ���� D������������Һ
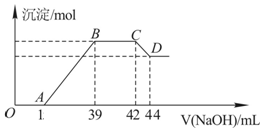
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���6mol•L-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
��1�������⣬��д���������������ϡ�����ᷴӦ�����ӷ���ʽ��8Fe+30H++3NO3-�T8 Fe3++3NH4++9 H2O
��2��ͼ��OA��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪH++OH-�TH2O��
��3����BC�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪNH4++OH-�TNH3•H2O��
��4������������Ԫ�ص����ʵ���Ϊ0.012mol��
��5��B���Ӧ�ij��������ʵ���Ϊ0.048mol��A���Ӧ������������Һ�����Ϊ15mL��
| A�� | �����ԣ�Cl2������ | B�� | ���ʷе㣺���ף�C12 | ||
| C�� | �⻯���ȶ��ԣ�HC1��PH3 | D�� | ���ԣ�HClO4��H3PO4 |
 ijѧϰС��ͬѧ������ͼװ������֤ͬ����Ԫ�طǽ����Եı仯���ɣ�
ijѧϰС��ͬѧ������ͼװ������֤ͬ����Ԫ�طǽ����Եı仯���ɣ�