��Ŀ����
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����
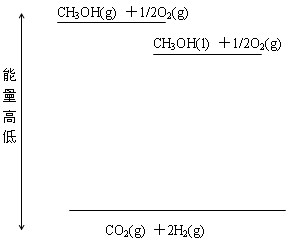
��CH3OH(g)��H2O(g) �� CO2(g)��3H2(g)��DH���� 49.0 kJ��mol��1
��CH3OH(g)��1/2O2(g) �� CO2(g)��2H2(g)��DH ����192.9 kJ��mol��1 ����˵����ȷ����
��CH3OH(g)��H2O(g) �� CO2(g)��3H2(g)��DH���� 49.0 kJ��mol��1
��CH3OH(g)��1/2O2(g) �� CO2(g)��2H2(g)��DH ����192.9 kJ��mol��1 ����˵����ȷ����
| A��CH3OH��ȼ����Ϊ192.9 kJ��mol��1 |
| B��CH3OH��ȼ����Ϊ133��9 kJ��mol��1 |
| C��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| D�����ݢ���֪��Ӧ��CH3OH(l) ��1/2O2(g) �� CO2(g) ��2H2(g)��DH ����192.9 kJ��mol��1 |
D
��νȼ������ָ1mol������ȫȼ�������ȶ������������ų�������������������ʽ֪��CH3OHת���H2�Ĺ��̿����Ƿ��ȡ�Ҳ���������ȷ�Ӧ������ʾ��Ӧ������ʾȼ���ȡ�������̬�ļ״�����������Һ̬�״�������ͼ�������Կ��Ƴ�Dѡ����ȷ��


��ϰ��ϵ�д�
�����Ŀ

 O2(g)===H2O(1) ��H=��285.6kJ/mol
O2(g)===H2O(1) ��H=��285.6kJ/mol
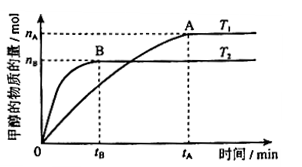
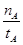 mol��L-1��min-1
mol��L-1��min-1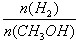 ����
����