��Ŀ����
�����10�֣����ס�������������ͬ���칹�壬�ڿ�����ȼ�յõ����������������ʱ����P4O6����������������P4O10��
��1����֪298Kʱ���ס�������ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
P4(s,����)+5O2(g)=P4O10(s) ��H1=" -2983.2" kJ?mol-1,
P(s������)+ 5/4O2(g)="1/4" P4O10(s) ��H2=" -738.5" kJ?mol-1
����¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ ��
��2����֪298Kʱ���ײ���ȫȼ�յ��Ȼ�ѧ����ʽΪP4(s,����)+3O2(g)=P4O6(s) ��H= -1638kJ?mol-1����ij�ܱ������м���62g����50.4L��������״���£�����������ʹ֮ǡ����ȫ��Ӧ�������õ���P4O10��P4O6�����ʵ���֮��Ϊ ����Ӧ�����зų�������ΪΪ ��
��3����֪����PCl3�ķ��ӽṹ��ͼ��ʾ�����ṩ���µĻ�ѧ���ļ��ܣ�KJ��mol��:P-P 198��Cl-Cl 243��P-Cl 331��
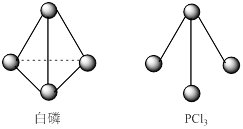
��ӦP4(s,����)+6Cl2(g)=4PCl3(s)�ķ�Ӧ�Ȧ�H = ��
��1����֪298Kʱ���ס�������ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
P4(s,����)+5O2(g)=P4O10(s) ��H1=" -2983.2" kJ?mol-1,
P(s������)+ 5/4O2(g)="1/4" P4O10(s) ��H2=" -738.5" kJ?mol-1
����¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ ��
��2����֪298Kʱ���ײ���ȫȼ�յ��Ȼ�ѧ����ʽΪP4(s,����)+3O2(g)=P4O6(s) ��H= -1638kJ?mol-1����ij�ܱ������м���62g����50.4L��������״���£�����������ʹ֮ǡ����ȫ��Ӧ�������õ���P4O10��P4O6�����ʵ���֮��Ϊ ����Ӧ�����зų�������ΪΪ ��
��3����֪����PCl3�ķ��ӽṹ��ͼ��ʾ�����ṩ���µĻ�ѧ���ļ��ܣ�KJ��mol��:P-P 198��Cl-Cl 243��P-Cl 331��

��ӦP4(s,����)+6Cl2(g)=4PCl3(s)�ķ�Ӧ�Ȧ�H = ��
�����10�֣�P4(s,����) = 4P(s������) ��H= ��29.2 kJ?mol-1��2�֣�
��2��3:1 ��3�֣� ��1323.45 kJ ��2�֣���3��-1326kJ?mol-1��3�֣�
��2��3:1 ��3�֣� ��1323.45 kJ ��2�֣���3��-1326kJ?mol-1��3�֣�
��1�����ݸ�˹���ɿ�֪���٣��ڡ�4���õ�P4(s,����) = 4P(s������) �����Ԧ�H=��2983.2 kJ?mol-1��738.5 kJ?mol-1��4����29.2 kJ?mol-1��
��2�������õ���P4O10��P4O6�����ʵ����ֱ���x��y��50.4L��������״���£���2.25mol��62g������0.5mol����x��y��0.5mol��3y��5x��2.25�����x��0.375mol��y��0.125mol�����Զ��ߵ����ʵ���֮����3�U1�����շų���������2983.2 kJ?mol-1��0.375mol��1638kJ?mol-1��0.125mol��1323.45kJ��
��3����Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ�����Ը÷�Ӧ�ķ�Ӧ����198kJ/mol��6��6��243kJ/mol��4��3��331kJ/mol����1326kJ/mol��
��2�������õ���P4O10��P4O6�����ʵ����ֱ���x��y��50.4L��������״���£���2.25mol��62g������0.5mol����x��y��0.5mol��3y��5x��2.25�����x��0.375mol��y��0.125mol�����Զ��ߵ����ʵ���֮����3�U1�����շų���������2983.2 kJ?mol-1��0.375mol��1638kJ?mol-1��0.125mol��1323.45kJ��
��3����Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ�����Ը÷�Ӧ�ķ�Ӧ����198kJ/mol��6��6��243kJ/mol��4��3��331kJ/mol����1326kJ/mol��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 H+ + CN���� K��1���� =" 4.9" ��10��10
H+ + CN���� K��1���� =" 4.9" ��10��10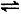 2SO3(g) ?��=��197kJ/mol���ڴ��¶��£���ס��������̶��ݻ����ܱ������зֱ�ͨ��2molSO2��1molO2��1mol SO2��0.5molO2����Ӧ�ﵽƽ��״̬ʱ�ų��������ֱ�ΪQ����Q���������й�ϵ��ȷ����
2SO3(g) ?��=��197kJ/mol���ڴ��¶��£���ס��������̶��ݻ����ܱ������зֱ�ͨ��2molSO2��1molO2��1mol SO2��0.5molO2����Ӧ�ﵽƽ��״̬ʱ�ų��������ֱ�ΪQ����Q���������й�ϵ��ȷ����
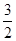 O2��g��=== Al2O3(s) ��H=" -1" 644.3 kJ? mol-1
O2��g��=== Al2O3(s) ��H=" -1" 644.3 kJ? mol-1