��Ŀ����
��13�֣���ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2(g)��CO(g)��CH3OH(l)��ȼ���ȡ�H�ֱ�Ϊ?285.8kJ��mol-1��?283.0kJ��mol-1��?726.5kJ��mol-1��
��ش��������⣺
(1)��������̫���ֽܷ�10molҺ̬ˮ���ĵ�������_____________kJ��
(2)�״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ___________��
(3)���ݻ�Ϊ2L���ܱ������У���CO��H2�ϳɼ״����������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ��ע��T1��T2������300�棩������˵����ȷ����_______������ţ�
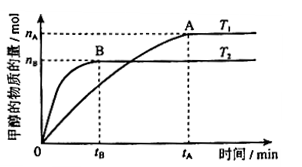
���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���ƽ������Ϊv(CH3OH)=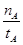 mol��L-1��min-1
mol��L-1��min-1
�ڸ÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ��С
�۸÷�ӦΪ���ȷ�Ӧ
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2���ﵽƽ��ʱ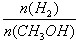 ����
����
��ش��������⣺
(1)��������̫���ֽܷ�10molҺ̬ˮ���ĵ�������_____________kJ��
(2)�״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ___________��
(3)���ݻ�Ϊ2L���ܱ������У���CO��H2�ϳɼ״����������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ��ע��T1��T2������300�棩������˵����ȷ����_______������ţ�

���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���ƽ������Ϊv(CH3OH)=
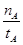 mol��L-1��min-1
mol��L-1��min-1�ڸ÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ��С
�۸÷�ӦΪ���ȷ�Ӧ
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2���ﵽƽ��ʱ
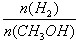 ����
����(1)2858 ��4�֣�(2) CH3OH(l)+O2(g)=CO(g)+2H2O(l) ��H =-443.5kJ��mol-1��5�֣�
(3)�ۢ� ��4�֣�
(3)�ۢ� ��4�֣�
��1������������ȼ���ȿ�֪���ֽ�10molˮ��Ҫ��������285.8kJ��mol-1��10mol��2858kJ��
��2��CO�ͼ״���ȫȼ�յķ���ʽ�ֱ�Ϊ��CO(g)��1/2O2(g)=CO2(g) ��H��?283.0kJ��mol-1����CH3OH(l)+3/2O2(g)=CO2(g)+2H2O(l) ��H =?726.5kJ��mol-1�����ݸ�˹���ɿ�֪���ڣ��ټ��õ�CH3OH(l)+O2(g)=CO(g)+2H2O(l)�����ԡ�H��?726.5kJ��mol-1��283.0kJ��mol-1����443.5kJ��mol-1��
��3��CO2��H2�ϳɼ״��Ļ�ѧ����ʽΪCO2(g)��3H2(g) CH3OH(g) + H2O(g)����ͼ���֪B�����ȵõ�ƽ�⣬����¶�T2��T1���¶ȸ�ƽ��ʱ�״������ʵ��������ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ��������ڼ״������ɣ�ƽ�ⳣ����С�����ڴ�����ȷ���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״������ʵ���Ϊ
CH3OH(g) + H2O(g)����ͼ���֪B�����ȵõ�ƽ�⣬����¶�T2��T1���¶ȸ�ƽ��ʱ�״������ʵ��������ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ��������ڼ״������ɣ�ƽ�ⳣ����С�����ڴ�����ȷ���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״������ʵ���Ϊ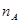 mol����ʱ�״���Ũ��Ϊ
mol����ʱ�״���Ũ��Ϊ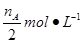 ���������ɼ״���ƽ������Ϊ��v(CH3OH)=
���������ɼ״���ƽ������Ϊ��v(CH3OH)=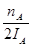 mol��L-1��min-1����ˢٲ���ȷ����Ϊ�¶�T2��T1������A��ķ�Ӧ��ϵ��T1�䵽T2ʱ��ƽ������淴Ӧ�����ƶ���������������Ũ�ȶ�����Ӧ��Ũ�ȣ����Ԣ���ȷ����ѡ)�ۢܡ�
mol��L-1��min-1����ˢٲ���ȷ����Ϊ�¶�T2��T1������A��ķ�Ӧ��ϵ��T1�䵽T2ʱ��ƽ������淴Ӧ�����ƶ���������������Ũ�ȶ�����Ӧ��Ũ�ȣ����Ԣ���ȷ����ѡ)�ۢܡ�
��2��CO�ͼ״���ȫȼ�յķ���ʽ�ֱ�Ϊ��CO(g)��1/2O2(g)=CO2(g) ��H��?283.0kJ��mol-1����CH3OH(l)+3/2O2(g)=CO2(g)+2H2O(l) ��H =?726.5kJ��mol-1�����ݸ�˹���ɿ�֪���ڣ��ټ��õ�CH3OH(l)+O2(g)=CO(g)+2H2O(l)�����ԡ�H��?726.5kJ��mol-1��283.0kJ��mol-1����443.5kJ��mol-1��
��3��CO2��H2�ϳɼ״��Ļ�ѧ����ʽΪCO2(g)��3H2(g)
 CH3OH(g) + H2O(g)����ͼ���֪B�����ȵõ�ƽ�⣬����¶�T2��T1���¶ȸ�ƽ��ʱ�״������ʵ��������ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ��������ڼ״������ɣ�ƽ�ⳣ����С�����ڴ�����ȷ���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״������ʵ���Ϊ
CH3OH(g) + H2O(g)����ͼ���֪B�����ȵõ�ƽ�⣬����¶�T2��T1���¶ȸ�ƽ��ʱ�״������ʵ��������ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ��������ڼ״������ɣ�ƽ�ⳣ����С�����ڴ�����ȷ���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״������ʵ���Ϊ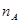 mol����ʱ�״���Ũ��Ϊ
mol����ʱ�״���Ũ��Ϊ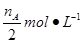 ���������ɼ״���ƽ������Ϊ��v(CH3OH)=
���������ɼ״���ƽ������Ϊ��v(CH3OH)=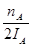 mol��L-1��min-1����ˢٲ���ȷ����Ϊ�¶�T2��T1������A��ķ�Ӧ��ϵ��T1�䵽T2ʱ��ƽ������淴Ӧ�����ƶ���������������Ũ�ȶ�����Ӧ��Ũ�ȣ����Ԣ���ȷ����ѡ)�ۢܡ�
mol��L-1��min-1����ˢٲ���ȷ����Ϊ�¶�T2��T1������A��ķ�Ӧ��ϵ��T1�䵽T2ʱ��ƽ������淴Ӧ�����ƶ���������������Ũ�ȶ�����Ӧ��Ũ�ȣ����Ԣ���ȷ����ѡ)�ۢܡ�
��ϰ��ϵ�д�
�����Ŀ
 2SO3(g) ?��=��197kJ/mol���ڴ��¶��£���ס��������̶��ݻ����ܱ������зֱ�ͨ��2molSO2��1molO2��1mol SO2��0.5molO2����Ӧ�ﵽƽ��״̬ʱ�ų��������ֱ�ΪQ����Q���������й�ϵ��ȷ����
2SO3(g) ?��=��197kJ/mol���ڴ��¶��£���ס��������̶��ݻ����ܱ������зֱ�ͨ��2molSO2��1molO2��1mol SO2��0.5molO2����Ӧ�ﵽƽ��״̬ʱ�ų��������ֱ�ΪQ����Q���������й�ϵ��ȷ����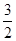 O2��g��=== Al2O3(s) ��H=" -1" 644.3 kJ? mol-1
O2��g��=== Al2O3(s) ��H=" -1" 644.3 kJ? mol-1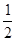 O2(g) == H2O(l) ��H3����285.8kJ��mol-1
O2(g) == H2O(l) ��H3����285.8kJ��mol-1
 Al2O3+Fe ��H��a kJ/mol
Al2O3+Fe ��H��a kJ/mol