��Ŀ����
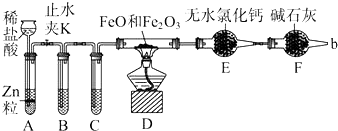
1��ij��ѧѧϰС����ʵ������̽����������Ũ����ķ�Ӧ����̽��������ͼ��ʾ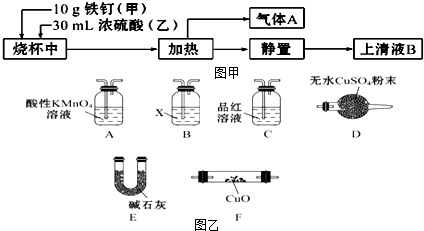
��1����ʵ�鰲ȫ�ĽǶȿ��ǣ�Ӧ�����ձ��м���ף���ס����ҡ�����
��2�������������⣬����ҺB�п��ܼȺ�Fe3+���ֺ�Fe2+��Ҫ��������ҺB������Fe2+��Ӧ������Լ���d������ĸ��ţ���
a��KSCN��Һ����ˮ b�����ۺ�KSCN��Һ c��NaOH��Һ d������KMnO4��Һ
��3������A����Ҫ�ɷ���SO2�������ܺ���H2��CO2������ͼ�С����ȡ�ʱ��������CO2��ԭ����C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$2SO2��+CO2��+2H2O���û�ѧ����ʽ��ʾ����
��4�����������������Լ�������A���Ƿ���H2��CO2�����еļг��������ܺͼ���װ���Ѿ���ȥ����������������˳����ACBEFDE������������ĸ���ţ���װ��A�������dz�ȥ����A�е�SO2��װ��B���Լ�X�Ļ�ѧʽ��Ca��OH��2��
���� ��1�������ձ��м��������ټ�Ũ���
��2��Fe2+���л�ԭ�ԣ���ʹ����KMnO4��Һ��ɫ��
��3�������к���������̼Ԫ�أ�C��Ũ���ᷴӦ���ɶ�����̼�����������ˮ��
��4����������A���Ƿ���H2��CO2��Ӧ���ȳ�ȥ��������Ȼ���ó���ʯ��ˮ���������̼���ٸ��Ȼ��ͨ��CuO����CuO��Ӧ����ˮ������ˮ����ͭ����ˮ�����ɣ�����֤����������
��� �⣺��1�������ձ��м��������ټ�Ũ���ᣬ����ȼ���Ũ���ᣬ�ټ���������������Ũ�������ˣ�
�ʴ�Ϊ���ף�
��2�������������⣬����ҺB�п��ܼȺ�Fe3+���ֺ�Fe2+��Fe2+���л�ԭ�ԣ���ʹ����KMnO4��Һ��ɫ������������KMnO4��Һ��������Fe2+��
�ʴ�Ϊ��d��
��3�������к���������̼Ԫ�أ�C��Ũ���ᷴӦ���ɶ�����̼�����������ˮ���䷴Ӧ�ķ���ʽΪ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$2SO2��+CO2��+2H2O��
�ʴ�Ϊ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$2SO2��+CO2��+2H2O��
��4����������A���Ƿ���H2��CO2��Ӧ���ȳ�ȥ�����������ѻ������ͨ������KMnO4��Һ����ͨ����̪��Һ������������Ƿ�������ȫ��Ȼ�������ͨ������ʯ��ˮ���ó���ʯ��ˮ���������̼����ͨ����ʯ�Ҹ��Ȼ��ͨ������CuO��������CuO��Ӧ����ˮ������ˮ����ͭ����ˮ�����ɣ�����֤������������������������˳����ACBEFDE��װ��A����ҺΪ����KMnO4��Һ�������dz�ȥ����A�е�SO2��װ��B�������������ڼ��������̼�����Լ�X�Ļ�ѧʽ��Ca��OH��2��
�ʴ�Ϊ��ACBEFDE����ȥ����A�е�SO2��Ca��OH��2��
���� ���⿼����Ũ��������ʡ�ʵ�鷽������������ۣ���Ŀ�Ѷ��еȣ�ע����������ʵ�鷽����Ƶ�ԭ�����۷�������ȷ������������ʼ����鷽���������ڿ���ѧ���ķ���������ʵ��������

| A�� | ���5.6LN2����n�������ӣ���NAһ��ԼΪ4n | |
| B�� | 18gˮ�������ĵ�������8NA | |
| C�� | ��0.5mol/L���Ȼ�����Һ�к���������Ϊ1.5NA | |
| D�� | 15gCH3+����8mol���� |
P4��s��+10Cl2��g���T4PCl5��g����H=b kJ•mol-1
P4������������ṹ��PCl5��P-Cl���ļ���Ϊc kJ•mol-1��PCl3��
P-Cl���ļ���Ϊ1.2c kJ•mol-1����������ȷ���ǣ�������
| A�� | P-P���ļ��ܴ���P-Cl���ļ��� | |
| B�� | ����Cl2��g��+PCl3��g���TPCl5��s���ķ�Ӧ�ȡ�H | |
| C�� | Cl-Cl���ļ���$\frac{b-a+5.6c}{4}$ kJ•mol-1 | |
| D�� | 1molP4��4molp-p�� |
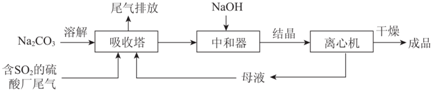
��1������˵����������ŷ�SO2���µĻ������⣺���꣮
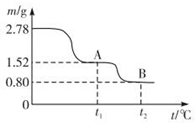
��2����ͼΪ��������Na2CO3��Һ��SO2��Ӧ��������Һ��ɱ仯������ڷ�Ӧ��ͼ��A����ǰ�������ӷ���ʽ��2CO32-+SO2+H2O=2HCO3-+SO32-��
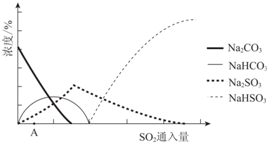
��3���к����з�������Ҫ��Ӧ�Ļ�ѧ����ʽ��NaHSO3+NaOH=Na2SO3+H2O��
| ������ʾ�� ��Na2SO3��33��ʱ�ܽ������䱥����Һ������33������ʱ�������ܽ�Ƚ��ͻ�������ˮNa2SO3����ȴ��33������ʱ����Na2SO3•7H2O�� ����ˮNa2SO3�ڿ����в��ױ�������Na2SO3•7H2O�ڿ������ױ������� |
������Na2SO3���ܽ�ƽ�����NaOH������ԭ��Na2SO3�����ܽ�ƽ�⣺Na2SO3��s��?2Na+ ��aq��+SO32- ��aq����NaOH����ʹc��Na+����������ƽ�������ƶ���
�ڽᾧʱӦѡ�����Ѳ�����B��ѡ����ĸ����
a��95��100�����������ֱ������
B��ά��95��100������Ũ�����д�����������
C��95��100�����Ũ������ȴ�����½ᾧ
��5��Ϊ����Na2SO3��Ʒ���Ƿ�����Na2SO4����ѡ�õ��Լ���ϡ���ᡢBaCl2��Һ��
��6��KIO3�ζ����ɲⶨ��Ʒ��Na2SO3�ĺ����������½�0.1260g ��Ʒ����ˮ�����������ָʾ������������KIO3����Һ��x mol/L�����еζ�����Һǡ������ɫ��Ϊ��ɫ������KIO3����Һ���Ϊy mL��
�ٵζ��յ�ǰ��Ӧ�����ӷ���ʽ�ǣ���IO3-+��SO32-=��3SO42-+��1I-��������ʽ����������
�ڳ�Ʒ��Na2SO3��M=126g/mol��������������3xy��100%��
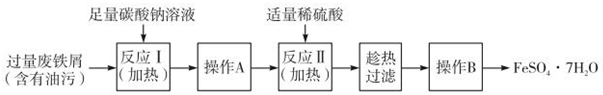
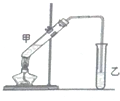 ��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
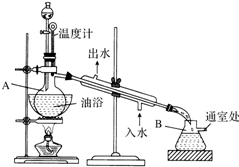 ������������ɫ����ˮ����ζ��Һ�壬�е�77.1�棬ij����ȡʵ���õ�������14.3mL��95%�Ҵ�23mL�����õ�ŨH2SO4������Na2CO3��Һ�Լ��������Ҵ���ϳ�����������Ȼ�����Һ����Ҫ����װ����ͼ��ʾ��ʵ�鲽���ǣ�
������������ɫ����ˮ����ζ��Һ�壬�е�77.1�棬ij����ȡʵ���õ�������14.3mL��95%�Ҵ�23mL�����õ�ŨH2SO4������Na2CO3��Һ�Լ��������Ҵ���ϳ�����������Ȼ�����Һ����Ҫ����װ����ͼ��ʾ��ʵ�鲽���ǣ�