��Ŀ����
14��ijͬѧ��ʵ����̽��NaHCO3�����ʣ������£�����0.10mol/LNaHCO3��Һ������pHΪ8.4��ȡ��������Һ�μ�CaCl2��Һ��pH=7���μӹ����в�����ɫ��������������ų�������˵������ȷ���ǣ�������| A�� | NaHCO3��Һ�ʼ��Ե�ԭ����HCO3-��ˮ��̶ȴ��ڵ���̶� | |
| B�� | ����CaCl2�ٽ���HCO3-��ˮ�� | |
| C�� | ��Ӧ�����ӷ���ʽ��2HCO3-+Ca2+�TCaCO3��+H2CO3 | |
| D�� | ��Ӧ�����Һ�д��ڣ�c��Na+��+2c��Ca2+���Tc��HCO3-��+2c��CO32-��+c��Cl-�� |
���� A��NaHCO3���ܵ�������ˮ�⣻
B������CaCl2����CO32-��
C��HCO3-��Ca2+����CaCO3�������ӣ������ӽ��HCO3-����H2CO3��
D�����ݵ���غ������
��� �⣺A��NaHCO3���ܵ�������ˮ�⣬ˮ���Լ��ԣ����������ԣ�NaHCO3��Һ�ʼ��Ե�ԭ����HCO3-��ˮ��̶ȴ��ڵ���̶ȣ���A��ȷ��
B����Һ�д���HCO3-?CO32-+H+������CaCl2����CO32-���ٽ�HCO3-�ĵ��룬��B����
C��HCO3-��Ca2+����CaCO3�������ӣ������ӽ��HCO3-����H2CO3����Ӧ�����ӷ���ʽ��2HCO3-+Ca2+�TCaCO3��+H2CO3����C��ȷ��
D����Һ�д��ڵ���غ㣬c��Na+��+c��H+��+2c��Ca2+��=c��HCO3-��+2c��CO32-��+c��Cl-��+c��OH-������Һ��pH=7��c��H+��=c��OH-����c��Na+��+2c��Ca2+��=c��HCO3-��+2c��CO32-��+c��Cl-������D��ȷ��
��ѡB��
���� ���⿼���˵������Һ�з�Ӧʵ�ʣ������ܽ�ƽ�������Ӧ�ã��������Һ������Ũ�ȴ�С������غ㡢�����غ��֪ʶ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
4�����ֶ�����Ԫ��W��X��Y��Z��Ԫ�����ڱ��е����λ����ͼ��ʾ��W����̬�⻯���������ۺ����ᷴӦ�������ӻ�����ɴ˿�֪��������
| W | X | |
| Y | Z |
| A�� | X��Y��Z����Ԫ�ص�����⻯������ȶ�����Y | |
| B�� | W��Y��Z����Ԫ�ض�Ӧ�������ˮ����һ������ǿ�� | |
| C�� | W��XԪ�ص�����⻯�ﶼ�Ƿǵ���� | |
| D�� | ZԪ�صĵ����ڻ�ѧ��Ӧ��ֻ�ܱ��������� |
5�����з���ʽ��ʾ������һ���Ǵ�������ǣ�������
| A�� | C2H6 | B�� | C2H4Cl2 | C�� | C4H10 | D�� | ��C6H10O5��n����ά�أ� |
2�������£�ij��Һ����ˮ�����c��OH-��=1��10-3mol/L������Һ�����ǣ�������
���������Һ ���Ȼ�����Һ ������������Һ ������������Һ��
���������Һ ���Ȼ�����Һ ������������Һ ������������Һ��
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ۢ� |
9������������ȷ���ǣ�������
| A�� | NaOH�������������� | |
| B�� | CaO�ɷ�ֹ�±���ʳƷ�������� | |
| C�� | �ⵯ���õ���2H��3H��Ϊͬλ�� | |
| D�� | ��ˮ�м��뾻ˮ����������ʹ��ˮ���� |
19��ij�л���X�Ľṹ��ʽΪ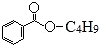 ������˵��������ǣ�������
������˵��������ǣ�������
 ������˵��������ǣ�������
������˵��������ǣ�������| A�� | X������ˮ | B�� | X�ķ���ʽΪC11H14O2 | ||
| C�� | ���Ϊ-C4H9��������3�� | D�� | X�ܷ����ӳɷ�Ӧ��ȡ����Ӧ |
3��NA��ʾ����٤������������˵����ȷ���ǣ�������
| A�� | 32g�������������ȫ��Ӧ�õ��ĵ�����Ϊ2NA | |
| B�� | ��1L2mol•L-1MgCl2��Һ�к��е�Cl-��Ϊ2NA | |
| C�� | ��״̬�£�2.24LCO2��2.24LH2O������ԭ������Ϊ0.3NA | |
| D�� | ��״���£�11.2L����������NaOH��Һ��Ӧת�Ƶĵ�����Ϊ0.5NA |
4��ij�л���ṹ��ʽΪ�� ����ϵͳ����������������������ȷ���ǣ�
����ϵͳ����������������������ȷ���ǣ�
 ����ϵͳ����������������������ȷ���ǣ�
����ϵͳ����������������������ȷ���ǣ�| A�� | �Զ��ױ� | B�� | 1��6-���ױ� | C�� | 1��2-���ױ� | D�� | �ұ� |
 ������Ҫ�Ļ���ԭ�ϣ���;�㷺��
������Ҫ�Ļ���ԭ�ϣ���;�㷺�� ��
��