��Ŀ����
ij�о�С����̽��SO2�Ļ�ѧ���ʣ����������ʵ�鷽����

��1��A�з�Ӧ�����ӷ���ʽΪ��
��2����B�м���SO2��Ư���ԣ���B����ʢ�Լ���Ϊ ��
��3����C��װH2S��Һ������SO2�������ԣ���C�з�Ӧ������ͻ�ѧ����ʽ�ֱ�Ϊ ��
��4����D��װ����Ư��Ũ��Һ��ͨ��SO2-�΅����D�г����˴�����ɫ������ͬѧ�Ƕ�ɫ�����ɷֽ�����̽������ش�����
����һ���ð�ɫ����ΪCaSO4
��������ð�ɫ����Ϊ
���������ð�ɫ����Ϊ�����������ʵĻ���
��5��E�������� ������©�������� ����E��Ϊ���Ը�����أ���Ӧ�����ӷ���ʽΪ�� ��

��1��A�з�Ӧ�����ӷ���ʽΪ��
��2����B�м���SO2��Ư���ԣ���B����ʢ�Լ���Ϊ
��3����C��װH2S��Һ������SO2�������ԣ���C�з�Ӧ������ͻ�ѧ����ʽ�ֱ�Ϊ
��4����D��װ����Ư��Ũ��Һ��ͨ��SO2-�΅����D�г����˴�����ɫ������ͬѧ�Ƕ�ɫ�����ɷֽ�����̽������ش�����
����һ���ð�ɫ����ΪCaSO4
��������ð�ɫ����Ϊ
���������ð�ɫ����Ϊ�����������ʵĻ���
��5��E��������
���㣺����ʵ�鷽�������
ר�⣺ʵ�������
��������1������������Ũ���ᷴӦ���������ơ����������ˮ�����ӷ���ʽ��Ũ������Ҫ������ѧʽ��
��2��SO2����Ư���ԣ���ʹƷ����ɫ��
��3���������������ⷴӦ���ɻ�ɫ�����ʣ�������������������ԣ��ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��4�������������Դ��ڴ����ᣬ�����������Ʒ�Ӧ����������ƣ�
��5�����������ж�����Ҫʹ��β������װ�ã����õ�©�����Է�ֹ���������Ը��������Һ�ܹ��������������������ᣬ�ݴ�д����Ӧ�����ӷ���ʽ��
��2��SO2����Ư���ԣ���ʹƷ����ɫ��
��3���������������ⷴӦ���ɻ�ɫ�����ʣ�������������������ԣ��ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��4�������������Դ��ڴ����ᣬ�����������Ʒ�Ӧ����������ƣ�
��5�����������ж�����Ҫʹ��β������װ�ã����õ�©�����Է�ֹ���������Ը��������Һ�ܹ��������������������ᣬ�ݴ�д����Ӧ�����ӷ���ʽ��
���
�⣺��1��A��Ũ����������������Һ��Ӧ���ɶ����������壬��Ӧ�����ӷ���ʽΪ��H2SO4��Ũ��+SO32-=SO42-+H2O+SO2����
�ʴ�Ϊ��H2SO4��Ũ��+SO32-=SO42-+H2O+SO2����
��2��SO2����Ư���ԣ���ʹƷ����ɫ������B��Ӧʢ��Ʒ����Һ��
�ʴ�Ϊ��Ʒ����Һ��
��3������ˮ��Һ�е���Ԫ�ض�Ϊ-2�ۣ����������Ӧ�����ϼۻ����ߣ�������������������������ԣ���Ӧ�Ļ�ѧ����ʽΪ��2H2S+SO2=3S+2H2O�У�����C�г��ֻ�ɫ������֤��SO2���������ԣ�
�ʴ�Ϊ���л�ɫ�������ɣ�2H2S+SO2=3S+2H2O��
��4�����������ܹ��������Ʒ�Ӧ����������ƣ����Լ����Ϊ����ɫ��������ΪCaSO3��
�ʴ�Ϊ��CaSO3��
��5�������������ж����壬����Ķ���������Ҫ��β������װ�ô�������������������ˮ�����������������������ն�������ʱ��Ҫʹ�õ��õ�©�����Ա��ֹ������������
���Ը��������Һ�����������������ԭ��Ӧ�����������̡����ᣬֻ�ж�������ˮ�����ӷ�Ӧ�б�����ѧʽ���ɵ��ӡ�����غ��֪���ӷ�ӦΪ5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+��
�ʴ�Ϊ�����ն�������ֹ��Ⱦ��������ֹ������5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+��
�ʴ�Ϊ��H2SO4��Ũ��+SO32-=SO42-+H2O+SO2����
��2��SO2����Ư���ԣ���ʹƷ����ɫ������B��Ӧʢ��Ʒ����Һ��
�ʴ�Ϊ��Ʒ����Һ��
��3������ˮ��Һ�е���Ԫ�ض�Ϊ-2�ۣ����������Ӧ�����ϼۻ����ߣ�������������������������ԣ���Ӧ�Ļ�ѧ����ʽΪ��2H2S+SO2=3S+2H2O�У�����C�г��ֻ�ɫ������֤��SO2���������ԣ�
�ʴ�Ϊ���л�ɫ�������ɣ�2H2S+SO2=3S+2H2O��
��4�����������ܹ��������Ʒ�Ӧ����������ƣ����Լ����Ϊ����ɫ��������ΪCaSO3��
�ʴ�Ϊ��CaSO3��
��5�������������ж����壬����Ķ���������Ҫ��β������װ�ô�������������������ˮ�����������������������ն�������ʱ��Ҫʹ�õ��õ�©�����Ա��ֹ������������
���Ը��������Һ�����������������ԭ��Ӧ�����������̡����ᣬֻ�ж�������ˮ�����ӷ�Ӧ�б�����ѧʽ���ɵ��ӡ�����غ��֪���ӷ�ӦΪ5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+��
�ʴ�Ϊ�����ն�������ֹ��Ⱦ��������ֹ������5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+��
���������⿼��������ʵ�鷽������ƣ����ؿ����������Ļ�ѧ���ʼ����鷽������Ŀ�Ѷ��еȣ�ע�����ն�������Ļ�ѧ���ʼ����鷽������ȷ����ʵ�鷽�������ԭ�������ֿ�����ѧ���ķ�����������������ѧʵ��������

��ϰ��ϵ�д�
�����Ŀ
���л�ѧ��ҵ�й��豸��ԭ�ϡ���Ӧ���������ǣ�������
| A���Ӵ������������¯��������V2O5��400��-500�� |
| B�������Ƽ�ƴ����������̼������ʳ�Ρ�������̼������30��-35��İ�������ʳ��ˮ���ն�����̼ |
| C���ϳɰ�������¯����̿������ý��500�� |
| D����������������ϳ���������������Ͻ�800�� |


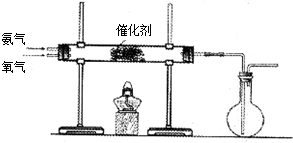
 ʱ��A�����ķ�Ӧ����Ϊ
ʱ��A�����ķ�Ӧ����Ϊ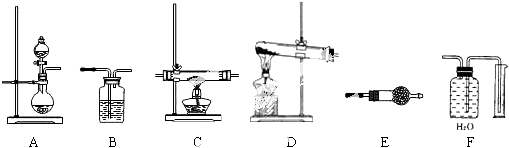


 ������Դ�뻷������Խ��Խ�����ǹ�ע��̼һ��ѧ��C1��ѧ����Ϊ�о����ȵ㣮��̼һ��ѧ�����Ե���̼��CO��CO2��CH4��CH3OH�Ⱥ�һ��̼ԭ�ӵ�����Ϊԭ�Ϻϳɹ�ҵ��Ʒ�Ļ�ѧ�빤�գ�
������Դ�뻷������Խ��Խ�����ǹ�ע��̼һ��ѧ��C1��ѧ����Ϊ�о����ȵ㣮��̼һ��ѧ�����Ե���̼��CO��CO2��CH4��CH3OH�Ⱥ�һ��̼ԭ�ӵ�����Ϊԭ�Ϻϳɹ�ҵ��Ʒ�Ļ�ѧ�빤�գ�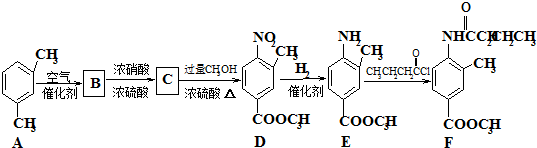
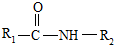 ��һ�������¿�ˮ��Ϊ
��һ�������¿�ˮ��Ϊ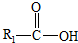 ��R2-NH2����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ��
��R2-NH2����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ��

