��Ŀ����
 ��1����������HA��������NaA�Ļ����Һ���ڻ�ѧ������������Һ�������м�����������ʱ����Һ������Ա仯�����ֽ�0.04mol?L-1HA��Һ��0.02mol?L-1NaOH��Һ�������ϣ��õ�������Һ��
��1����������HA��������NaA�Ļ����Һ���ڻ�ѧ������������Һ�������м�����������ʱ����Һ������Ա仯�����ֽ�0.04mol?L-1HA��Һ��0.02mol?L-1NaOH��Һ�������ϣ��õ�������Һ������HAΪHCN������Һ�Լ��ԣ�����Һ��c��CN-��
����HAΪCH3COOH������Һ�����ԣ���Һ�����е����Ӱ�Ũ���ɴ�С���е�˳����
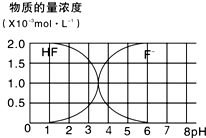
��2��25��ʱ��2.0��10-3mol?L-1HF��Һ�У�������ҺpH��������Һ����仯���õ��ģ�HF����c��F-������ҺpH�ı仯��ϵ��ͼ������4.0��10-4mol?L-1CaCl2��Һ��4.0��10-3mol?L-1HF��Һ�������ϣ����ڻ��ҺpH=4�����Ե���ʱ���Һ����ı仯����ͨ����ʽ����˵���Ƿ���CaF2����������[��֪Ksp��CaF2����1.5��10-10]��
���㣺�����ʱ�Ķ����жϼ��й�ph�ļ���,���ܵ���ʵ��ܽ�ƽ�⼰����ת���ı���
ר�⣺
��������1���ٴ���Һ�����ԵĽǶȱȽ�����Ũ�ȴ�С��
����HAΪCH3COOH������Һ�����ԣ�˵��c��H+����c��OH-���������Һ������ԭ�������
��2����ͼ��PH=4ʱ����Һ��c��F-��=1.6��10-3mol?L-1������Qc=c��Ca2+��c2��F-�����㣬Ȼ����ݼ������ж��Ƿ��г������ɣ�
����HAΪCH3COOH������Һ�����ԣ�˵��c��H+����c��OH-���������Һ������ԭ�������
��2����ͼ��PH=4ʱ����Һ��c��F-��=1.6��10-3mol?L-1������Qc=c��Ca2+��c2��F-�����㣬Ȼ����ݼ������ж��Ƿ��г������ɣ�
���
�⣺��1���ٸ���Һ�Լ��ԣ���c��H+����c��OH-����������Һ������ԭ���֪c��Na+��+c��H+��=C��CN-��+c��OH-������c��Na+����c��CN-����
�ʴ�Ϊ��������Ϊc��Na+��+c��H+��=C��CN-��+c��OH-������Һ�Լ��ԣ���c��H+����c��OH-��������c��Na+����c��CN-����
����HAΪCH3COOH������Һ�����ԣ�˵��c��H+����c��OH-����������Һ������ԭ���֪c��CH3COO-����c��Na+����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
��2������ͼ֪����pH=4ʱ����Һ��c��F-��=1.6��10-3mol/L��Qc=c��Ca2+����c2��F-��=2.0��10-3����1.6��10-3��2=5.12��10-10��Ksp��CaF2���������г������ɣ�
�ʴ�Ϊ����pH=4ʱ��Qc=c��Ca2+��?c2��F-��=2.0��10-3����1.6��10-3��2=5.12��10-10��Ksp��CaF2���������г������ɣ�
�ʴ�Ϊ��������Ϊc��Na+��+c��H+��=C��CN-��+c��OH-������Һ�Լ��ԣ���c��H+����c��OH-��������c��Na+����c��CN-����
����HAΪCH3COOH������Һ�����ԣ�˵��c��H+����c��OH-����������Һ������ԭ���֪c��CH3COO-����c��Na+����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
��2������ͼ֪����pH=4ʱ����Һ��c��F-��=1.6��10-3mol/L��Qc=c��Ca2+����c2��F-��=2.0��10-3����1.6��10-3��2=5.12��10-10��Ksp��CaF2���������г������ɣ�
�ʴ�Ϊ����pH=4ʱ��Qc=c��Ca2+��?c2��F-��=2.0��10-3����1.6��10-3��2=5.12��10-10��Ksp��CaF2���������г������ɣ�
���������⿼��������ϵĶ����жϡ���Һ������Ũ�ȶ��ԱȽϼ������ܽ�ƽ��ļ����֪ʶ����Ŀ�Ѷ��еȣ�����֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ�����������������ѧ����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
������������������Һ���ȣ��ȿ�������ϩ�ֿ������Ҵ��������������ǣ�������
| A��������ϩ�����ȵ��������ص�ˮ��Һ |
| B�������Ҵ������ȵ��������ص�ˮ��Һ |
| C��������ϩ������170���½��е� |
| D�������Ҵ������ȵ��������صĴ���Һ |
���з�Ӧ���û���ֱ���Ƶõ��ǣ�������
��FeCl2��FeCl3��Fe��OH��3��Fe��OH��2��Cu2S��
��FeCl2��FeCl3��Fe��OH��3��Fe��OH��2��Cu2S��
| A���٢ڢۢ� | B���ڢ� |
| C���ڢۢ� | D��ȫ�� |
������������ص�����������ǣ�������
| A�����Գ���ˮ��AOW������������HlNl��������Ϊ��������ǿ������ |
| B��ˮ�Ĵ������õ�Ư�ۺ����������ߵ�����ԭ����ͬ |
| C�����������������Լ��������������������������Ҫ��� |
| D���ع��͵���Ҫ�ɷ�����֬������������͡�ú�Ͳ���ͬ |
��0.2mol?L-1HCN��Һ��0.1mol?L-1��NaOH��Һ�������Ϻ���Һ�Լ��ԣ����й�ϵʽ����ȷ���ǣ�������
| A��c ��HCN����c ��CN-�� |
| B��c ��Na+����c ��CN-�� |
| C��c ��HCN��-c ��CN-��=c ��OH-�� |
| D��c ��HCN��+c ��CN-��=0.1mol?L-1 |
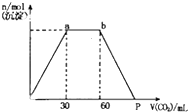 ��Ba��OH��2��KOH���Һ�л���ͨ��CO2���������������ɳ������ʵ�����ͨ��CO2�������VmL�Ĺ�ϵ��ͼ��ʾ�����н�������ȷ���ǣ�������
��Ba��OH��2��KOH���Һ�л���ͨ��CO2���������������ɳ������ʵ�����ͨ��CO2�������VmL�Ĺ�ϵ��ͼ��ʾ�����н�������ȷ���ǣ�������| A��ԭ�������n[Ba��OH��2]��n��KOH��=1��2 |
| B��p������Ϊ120mL |
| C��p����Һ������ΪBa��HCO3��2 |
| D��a��b�η�Ӧ�ֶ��Σ����ӷ���ʽΪ��CO2+2OH-=CO32-+H2O CO32-+H2O+CO2=2HCO3- |