��Ŀ����
18�������£�������Һ�е���Ũ�ȹ�ϵ��ȷ���ǣ�������| A�� | ��Na2CO3��Һ��һ���У�c��Na+��=2c��CO32-��+2c��HCO3-��+2c��H2CO3�� | |
| B�� | ��NaHCO3��Һ��һ���У�c��Na+��+c��H+��=c��HCO3-��+c��CO32-��+c��OH-�� | |
| C�� | ��NH4Cl��Һ��һ���У�c��H+��=c��NH3•H2O��+c��OH-�� | |
| D�� | ��CH3COONa��Һ��һ���У�c��Na+����c��OH-����c��CH3COO-����c��H+�� |
���� A��̼������Һ�д��������غ�n��Na��=2n��C����
B��̼��������Һ�д��ڵ���غ���������������������������ͬ��
C���Ȼ����Һ�д��������غ㣬��Һ��ˮ�����������������Ũ�Ⱥ��������������д�����ʽ��Ũ���ܺ���ͬ��
D����CH3COONa��Һ�д��������ˮ����Һ�Լ��ԣ�
��� �⣺A��̼������Һ�д��������غ�n��Na��=2n��C������Na2CO3��Һ��һ���У�c��Na+��=2c��CO32-��+2c��HCO3-��+2c��H2CO3������A��ȷ��
B��̼��������Һ�д��ڵ���غ㣬���������������������ͬ����NaHCO3��Һ��һ���У�c��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-������B����
C���Ȼ����Һ�д��������غ㣬��Һ��ˮ�����������������Ũ�Ⱥ��������������д�����ʽ��Ũ���ܺ���ͬ����NH4Cl��Һ��һ���У�c��H+��=c��NH3•H2O��+c��OH-������C��ȷ��
D����CH3COONa��Һ�д��������ˮ����Һ�Լ��ԣ���CH3COONa��Һ��һ���У�c��Na+����c��CH3COO-����c��OH-����c��H+������D����
��ѡAC��
���� ���⿼������Ũ�ȴ�С�ıȽϣ���Ŀ�Ѷ��еȣ���ȷ����غ㡢�����غ㡢�ε�ˮ��ԭ���ĺ���Ϊ���ؼ�������������ѧ���ķ������������������Ӧ�û���֪ʶ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�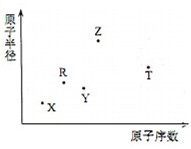 ����������Ԫ��X��Y��Z��R��T��ԭ�Ӱ뾶��ԭ��������ϵ��ͼ��ʾ��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T�γɵĻ�����Z2T���ƻ�ˮ�ĵ���ƽ�⣮�����ƶ���ȷ���ǣ�������
����������Ԫ��X��Y��Z��R��T��ԭ�Ӱ뾶��ԭ��������ϵ��ͼ��ʾ��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T�γɵĻ�����Z2T���ƻ�ˮ�ĵ���ƽ�⣮�����ƶ���ȷ���ǣ�������| A�� | ԭ�Ӱ뾶�����Ӱ뾶�����㣺Y��Z | |
| B�� | �⻯��ķе㲻һ���ǣ�Y��R | |
| C�� | ����������Ӧˮ��������ԣ�T��R | |
| D�� | ��X��R��Y��Z����Ԫ����ɵĻ�����ˮ��Һһ���Լ��� |
| A�� | NaOH | B�� | Na2O | C�� | Na | D�� | CaO |
| A�� | ��ˮ��Һ�ȶ������������ӣ���Ϊ����� | |
| B�� | SiO2��Al2O3���ȿ��������ֿ�����Ӧ���ʶ������������� | |
| C�� | ��������Һ�ķ�ɢ�����Ӿ�����ͨ����ֽ | |
| D�� | ���������ڿ�ʴ������������SiO2��������������� |
| A�� | Si��P��S��ClԪ�صĵ�������������Խ��Խ���� | |
| B�� | �ǽ���Ԫ�صķǽ�����Խǿ�����������Ӧˮ���������Ҳһ��Խǿ | |
| C�� | Ԫ��ԭ������������Խ�࣬Ԫ�ؽ�����Խǿ | |
| D�� | F-��O2-��Mg2+��Na+���Ӱ뾶��С |
 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų����������ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų����������ش��������⣺

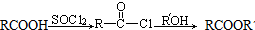 ��R��R�@����������
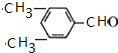
��R��R�@����������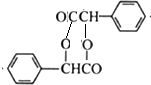 ��������C�еĺ��������ŵ��������Ȼ���
��������C�еĺ��������ŵ��������Ȼ��� ��
�� ��
��
