��Ŀ����
1��ij��ѧ�о���ѧϰС��Ե������Һ�����µĹ����ܽᣨ���ڳ����£���������ȷ���ǣ���������pH=1��ǿ����Һ����ˮϡ�ͺ���Һ������Ũ�Ⱦ�����
�ڵ����ʵ���Ũ�ȵ�Na2CO3��Һ��NaHCO3��Һ�������ϣ�������Һ�У�
c��CO32-��+2c��OH-��=2c��H+��+c��HCO3-��+3c��H2CO3��
��pH��ȵ�������Һ��a��CH3COONa��b��C6H5ONa��c��NaHCO3��d��NaOH������֪���ԣ�C6H5OH��H2CO3������Һ���ʵ���Ũ����С�����˳��Ϊd��b��c��a
��1L 0.1mol/L NH4NO3��Һ�е�ԭ����С��0.2NA
��pH=4.75��Ũ�Ⱦ�Ϊ0.1mol•L-1��CH3COOH��CH3COONa�����Һ��
c��CH3COO-��+c��OH-����c��CH3COOH��+c��H+��
�ס�������Һ����ǿ����ʣ���֪����Һ��pH������ҺpH����������ס�������Һ�������ϣ����û��Һ��pH���ܵ���7
�߰�0.1mol/L��NaHCO3��Һ��0.3mol/L��Ba��OH��2��Һ�������ϣ�������Һ��һ�����ڣ�c��OH-����c��Ba2+����c��Na+����c��H+��
����pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�У�ˮ�ĵ���̶Ȳ�ͬ��
| A�� | �٢ڢۢ� | B�� | �ۢݢޢ� | C�� | �٢ۢܢ� | D�� | �ڢۢޢ� |
���� ��pH=1��ǿ����Һ����ˮϡ�ͺ���Һ��c��H+�����������Ũ�ȶ���С�����¶Ȳ��䣬���ӻ��������䣬�ݴ��ж�c��OH-���仯��
���κε������Һ�ж����ڵ���غ�������غ㣬���ݵ���غ�������غ��жϣ�
��NaOH��ǿ�pH��ͬʱ����Ũ����С���������ˮ��̶�Խ��pH��ͬ��������ҺŨ��ԽС��
�ܸ���ԭ���غ��ж�Nԭ�Ӹ�����
��pH=4.75��Ũ�Ⱦ�Ϊ0.1mol•L-1��CH3COOH��CH3COONa�����Һ�����ԣ�˵���������̶ȴ��ڴ��������ˮ��̶ȣ���������غ㡢����غ��жϣ�
����pH֮�͵���14���������Ͽɵ���7��
�߰�0.1mol/L��NaHCO3��Һ��0.3mol/L��Ba��OH��2��Һ�������ϣ����߷�����ӦNaHCO3+Ba��OH��2=BaCO3��+NaOH+H2O����Һ�е�������NaOH��Ba��OH��2�����ߵ����ʵ���Ũ�ȷֱ�Ϊ0.05mol/L��0.1mol/L���ٽ�������غ��жϣ�
������Һ�е�������������Һ�е������Ӿ�ȫ��������ˮ�ĵ��룮
��� �⣺��pH=1��ǿ����Һ����ˮϡ�ͺ���Һ��c��H+�����������Ũ�ȶ���С�����¶Ȳ��䣬���ӻ��������䣬�ݴ��ж�c��OH-�����ʴ���
�ڸ��ݻ����Һ�е������غ���У�2c��Na+��=3[c��CO32-��+c��HCO3-��+c��H2CO3��]�����������غ���У�c��Na+��+c��H+��=2c��CO32-��+c��HCO3-��+c��OH-��
���Եã�c��CO32-��+2c��OH-��=2c��H+��+c��HCO3-��+3c��H2CO3�����ʢ���ȷ��
�������ε�ˮ��Һ���Լ��ԣ�ͬŨ�ȣ�����ǿ��˳��Ϊd��b��c��a����pH��ȵ�������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a���ʢ���ȷ��
����Һ��NH4NO3�����ʵ���n=CV=0.1mol/L��1L=0.1mol����1mol������к�2mol��ԭ�ӣ���0.1mol������к�0.2mol��ԭ�Ӽ�0.2NA�����ʢܴ���
��pH=4.5Ũ�Ⱦ�Ϊ0.1mol•L-1��CH3COOH��CH3COONa�����Һ�У��������ˮ�⣬c��Na+����c��CH3COOH��������غ�c��CH3COO-��+c��OH-��=c��Na+��+c��H+������c��CH3COO-��+c��OH-����c��CH3COOH��+c��H+�����ʢݴ���
�����ߵ�pH֮�͵���14ʱ������ҺpH=7���ʢ���ȷ��
�߰�0.1mol/L��NaHCO3��Һ��0.3mol/L��Ba��OH��2��Һ�������ϣ����߷�����ӦNaHCO3+Ba��OH��2=BaCO3��+NaOH+H2O����Һ�е�������NaOH��Ba��OH��2�����ߵ����ʵ���Ũ�ȷֱ�Ϊ0.05mol/L��0.1mol/L�����������غ�ã������Һ������Ũ�ȴ�С˳����c��OH-����c��Ba2+����c��Na+����c��H+�����ʢ���ȷ��
������Һ�е�������������Һ�е������Ӿ�ȫ��������ˮ�ĵ��룬��pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�У�ˮ������������Ӻ���������Ϊ10-11mol/L����ˮ�ĵ���̶���ͬ���ʢ����
��ѡD��
���� ������ۺϣ��漰����ˮ�⡢������ʵĵ����֪ʶ�㣬���ؿ���ѧ�����������������ѵ����ж�����Ũ�ȴ�С��ע�����غ㼰�����غ��������ã���Ŀ�Ѷ��еȣ�

 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�| A�� | С��7 | B�� | ����7 | C�� | ����7 | D�� | ���ж� |
 �����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH�����
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH�����| ʵ���� | HA���ʵ���Ũ�� ��mol•L-1�� | NaOH���ʵ���Ũ�� ��mol•L-1�� | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=9 |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
��1���ӵڢ�����������������ҺpH=9��ԭ����A-+H2O?HA+OH-�������ӷ���ʽ��ʾ�����������û����Һ����ˮ�������c��OH-��=10-5mol•L-1��
��2���ڢ������������c��0.2��ѡ���������������=�������û��Һ������Ũ��c��A-�� ��c��Na+�� �Ĵ�С��ϵ��c��A-��=c��Na+����
��3���ӵڢ���ʵ����������˵����ʱHA�ĵ���̶ȣ�NaA��ˮ��̶ȣ�ѡ���������������=�������û����Һ�и�����Ũ���ɴ�С��˳����c��A-����c��Na+����c��H+����c��OH-����
��4����һ��Ũ�ȵ�NaA��Һϡ��100��ʱ����pH�仯��ͼ A��B��C�����е�B������ĸ�����ߣ�
��5�����������漰�����������������������Һ��
�ף�0.1mol/LNaA
�ң�0.1mol/LNaA��0.1mol/LHA
����0.1mol/LHA
����0.1mol/LNaA��0.1mol/LNaOH
��A-����Ũ���ɴ�С��˳��Ϊ�ң������ף���������ţ���
| A�� | ��2-��-1-��ϩ����ͨ���Ӿ۷�Ӧ�Ƶ� | |
| B�� | ��2-��-1-��ϩ�ķ���ʽΪ ��C4H8��n | |
| C�� | ��2-��-1-��ϩ��ȫȼ������CO2��H2O�����ʵ������ | |
| D�� | ��2-��-1-��ϩ��ʹ������Ȼ�̼��Һ��ɫ |
| ʱ��/min | 0 | 10 | 20 | 40 | 50 | |
| T1 | n��CH4��/mol | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
| T2 | n��CH4��/mol | 0.50 | 0.30 | 0.18 | �� | 0.15 |
| A�� | T2ʱCH4��ƽ��ת����Ϊ70.0% | |
| B�� | �÷�Ӧ�ġ�H��0��T1��T2 | |
| C�� | ���������������䣬T1ʱ��ƽ����ϵ���ٳ���0.30 mol CH4��0.80 mol H2O��g����ƽ��������Ӧ�����ƶ� | |
| D�� | ���������������䣬T1ʱ��ƽ����ϵ���ٳ���0.50 mol CH4��1.20 mol NO2����ԭƽ����ȣ�����ƽ��ʱN2��Ũ���������������С |
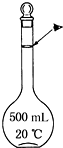 ʵ��������500mL 0.5mol•L-1��NaCl��Һ�������²������裺
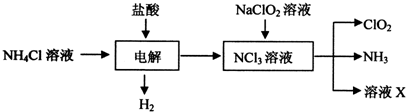
ʵ��������500mL 0.5mol•L-1��NaCl��Һ�������²������裺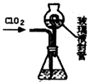 �������ȣ�C1O2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч����������������һ�ֻ���ɫ�����壬������ˮ��ʵ���ҿ���Fa-14Cl�����ᡢNaCl02���������ƣ�Ϊԭ���Ʊ�C1O2���������£�
�������ȣ�C1O2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч����������������һ�ֻ���ɫ�����壬������ˮ��ʵ���ҿ���Fa-14Cl�����ᡢNaCl02���������ƣ�Ϊԭ���Ʊ�C1O2���������£�