��Ŀ����
15�����м��־������ĺ���������밴Ҫ������
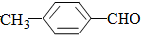
��1��-�������¢ٿ�ת��Ϊ�ܣ������Լ�����ʵ�ָ�ת������C������ĸ����
A�����������ҺB��H2C��������Һ/H+ D������������Һ
��2����д���������������Ģٵ�ͬ���칹��
 ��
��A�������г������ⲻ��������״�ṹ
B��������һ�ȴ���������
C��������ˮ�е�Br2��1��4���з�Ӧ
��3���������Ǿ����������������ʵ�ԭ�ӻ�ԭ���ţ����л�������л��ż���Ӱ��Ҳ�ᵼ���������ʵı仯�����ݴ��ֹ۵�ش��������⣺
�٢ڢ������л����ܽ����ɴ�С��˳��ڣ��ۣ��٣�����ĸ�������ǻ�Ϊͬϵ�
�����������顢�Ҷ����ķе��ɸߵ��͵�˳����������Ҷ��������飮
���ᡢ���ӡ��������������ǿ������˳���������ӣ�
���� ��1��-�������¢ٿ�ת��Ϊ�ܣ�����ȩ������������������������������Һ����������
��2��A�������г������ⲻ��������״�ṹ��B��������һ�ȴ��������֣���������3��Hԭ�ӿɷ��ϣ�C��������ˮ�е�Br2��1��4���з�Ӧ��������к��з��ǻ�����λ����λHԭ�ӿɱ�ȡ�����Һ���̼̼˫����
��3������ȩ����������̼ԭ����Խ�࣬��Ӧ�л�����ܽ��ԽС��
�ǻ���ĿԽ�࣬�л���е�Խ�ߣ����鳣����Ϊ��̬���ݴ��жϸ��л���ķе��С��
��������������˵����Ӱ�죬������������ǿ�����������С�ڱ����ᣮ
��� �⣺��1��-�������¢ٿ�ת��Ϊ�ܣ�����ȩ������������������������������Һ�������������и�����ؾ���ǿ�����ԣ���������������ֻ��C���ϣ�
�ʴ�Ϊ��C��
��2��A�������г������ⲻ��������״�ṹ��B��������һ�ȴ��������֣���������3��Hԭ�ӿɷ��ϣ�C��������ˮ�е�Br2��1��4���з�Ӧ��������к��з��ǻ�����λ����λHԭ�ӿɱ�ȡ�����Һ���̼̼˫�������Ӧ�Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3������ȩ���ı��������е�̼ԭ��Խ�࣬��Ӧ�л�����ܽ��ԽС�������ߵ��ܽ�ȴ�СΪ�� ��
�� ��
�� ��������Ϊͬϵ�
��������Ϊͬϵ�
�����ǻ���ĿԽ�࣬��ˮ�еķе�Խ�ߣ������ڳ�����Ϊ��̬�������ߵķе��СΪ�����������Ҷ��������飬
���ܱ�����Ӱ�죬����������Դ��ᣬ��������ʯ̼�ᣬ�����������������Դ�СΪ�������������ӣ�
�ʴ�Ϊ���ڣ��ۣ��٣�ͬϵ����������Ҷ��������飻�����������ӣ�
���� ���⿼�����л���ṹ�����ʣ�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ��漰�л���ṹ��ʽ��ȷ����ͬ���칹�����д���л���ṹ�����ʵ�֪ʶ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ���ѧ���ķ������������Ӧ��������

 ��ͼ��ʾ��m��ʾ��Һ�����ɳ��������ʵ�����n��ʾ��Һ�м������ʵ��������з�Ӧ�У��ܰ�����ͼ���߽��е��ǣ�������
��ͼ��ʾ��m��ʾ��Һ�����ɳ��������ʵ�����n��ʾ��Һ�м������ʵ��������з�Ӧ�У��ܰ�����ͼ���߽��е��ǣ�������| A�� | ��Ca��OH��2��KOH�Ļ����Һ��ͨ��CO2 | |
| B�� | ��AlCl3��NH4Cl�Ļ����Һ�м�NaOH��Һ | |
| C�� | ��Al2��SO4��3�ͣ�NH4��2SO4�Ļ����Һ�м�Ba��OH��2��Һ | |
| D�� | ��NH4Al��SO4��2��Һ�м���NaOH��Һ |
| A�� | 2.4g | B�� | 4.8g | C�� | 6g | D�� | 6.18g |
| A�� | ������20mL NaOH��Һʱ��2c��SO42-��=c��NH3•H2O��+c��NH4+�� | |
| B�� | ������30mL NaOH��Һʱ��pH��7����c��NH4+����c��NH3•H2O����c��OH-����c��H+�� | |
| C�� | ����Һ������ʱ��c��NH4+����c��SO42-����c��Na+����c��H+��=c��OH-�� | |
| D�� | ������Ӧ�����У�c��H+��+c��Na+��+c��NH4+��=c��OH-��+2c��SO42-�� |
| A�� | ������PM2.5��2.5�����µĿ�����Ĵ���һ���ܹ��γɶ����ЧӦ | |
| B�� | ������ɱ�����в�������Ϊ���ɲ����ĵ��������ȱ��� | |
| C�� | ����ϩʳƷ��װ����ʳ�ﱣ��Ĥ�������ĸ߷��ӻ�������� | |
| D�� | ˮ��ĸ�Ӫ�����뺬N��Pϴ�·۹㷺ʹ���й� |
| A�� | 1.5 g�������еĵ�����ĿΪ0.9NA | |
| B�� | ��1L0.1 mol•L-1Na2S��Һ�У���������������0.1NA | |
| C�� | 78g Na2O2������CO2��ȫ��Ӧ��ת�Ƶĵ�������ΪNA | |
| D�� | ��KIO3+6HI=KI+3I2+3H2O��Ӧ�У�ÿ����3 molI2����ת��6NA������ |
| A�� | ̫���ܹ��ص���Ҫ�ɷ��ǵ��ʹ� | |
| B�� | �˵�վй©�ķ����Ե�-131��${\;}_{53}^{131}$I�����-137��${\;}_{55}^{137}$Cs������ͬλ�� | |
| C�� | ����β����Ⱦ���к��еĵ�����������Ͳ���ȫȼ����ɵ� | |
| D�� | ij��ˮ��Ʒ����һ��ʱ���pH��4.68��Ϊ4.28������Ϊ������CO2 |