��Ŀ����
14��X��Y��Z��M��W��R��Ԫ�����ڱ���1��36��Ԫ�أ���ԭ��������������X��Y��Z��λ��ͬһ���ڵ�����Ԫ�أ�YԪ�ػ�̬ԭ�ӵ�2p������ڰ����״̬��MΪԪ�����ڱ�1��36��Ԫ���е縺����С��Ԫ�أ�WԪ�ػ�̬ԭ�ӵļ۵��ӹ���Ϊ3d74s2��Rλ�����ڱ���11�У��ش��������⣨�����ʾ����Ԫ�ر�������Ӧ��Ԫ�ط��ţ�����1��X��Y��Z����Ԫ�صĵ�һ�������ɴ�С��˳����N��O��C����Ԫ�ط��ű�ʾ����Y�������̬�⻯����ˮ�е��ܽ��Զ����X�������̬�⻯���Ҫԭ���Ƿ�����ˮ����֮���γ����������������ˮ���Ӷ��Ǽ��Է��ӣ��������ܣ�
��2��RԪ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1��Z��M�γɻ�����M2Z2�ĵ���ʽΪ
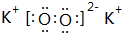 ��
����3��X��Z��W����Ԫ�ؿ����γ��ٺ�ɫ�������Ĺ��������W2��XZ��8������������ṩ�չ������Co���ṩ�¶Ե��ӵ���CO���ѧʽ����
��4����֪ij�����ﲿ�ֽṹ��ͼ��a����ʾ���û�������X��Y��Ԫ����ɣ�Ӳ�ȳ������ʯ���û�����Ļ�ѧʽΪC3N4���侧������Ϊԭ�Ӿ��壬������X��Y����Ԫ��ԭ�ӵ��ӻ���ʽ��Ϊsp3��

��5����ͼ��b���б��R������Rԭ�ӵ�λ�ã��þ�����Rԭ��ֱ��Ϊa pm��R�����ԭ������ΪM�������ӵ�����ΪNA���þ����ܶȱ���ʽΪ$\frac{\sqrt{2}M��1{0}^{30}}{{a}^{3}{N}_{A}}$g•cm-3����a��M��NA��ʾ����
���� X��Y��Z��M��W��R��Ԫ�����ڱ���1��36��Ԫ�أ���ԭ��������������YԪ�ػ�̬ԭ�ӵ�2P������ڰ����״̬��ԭ�Ӻ�������Ų�ʽΪ1s22s22p3����YΪNԪ�أ�X��Y��Z��λ��ͬһ���ڵ�����Ԫ�أ����ԭ��������֪XΪCԪ�ء�ZΪOԪ�أ�MΪԪ�����ڱ�1��36��Ԫ���е縺����С��Ԫ�أ���MΪK��WԪ�ػ�̬ԭ�ӵļ۵��ӹ���Ϊ3d74s2����WΪCo��Rλ�����ڱ���11�У�ԭ����������Co����RΪCu��
��1��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���A�塢��A��ֱ�Ϊȫ���������ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ�
NH3������ˮ����֮���γ����������������ˮ���Ӷ��Ǽ��Է��ӣ��������ܣ�ʹ������ˮ�е��ܽ��Զ���ڼ���ģ�
��2��RΪCu�����������Ϊ29�������������ԭ����дԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ��Z��M�γɻ�����M2Z2ΪK2O2���ɼ���������������ӹ��ɣ�
��3������ԭ�ӻ����Ӻ��пչ�������庬�й¶Ե��ӣ�
��4���Ӹ����ġ�������Ľṹͼ��֪��Cԭ���γ�4������Nԭ���γ�3�������û�����Ļ�ѧʽΪC3N4�����仯ѧ��Ϊ���ۼ������Ӳ�ȴ��֪��Ϊԭ�Ӿ��壬̼ԭ���γ�4����������Nԭ���γ�3������������1�Թ¶Ե��ӣ��۲���Ӷ�������4��
��5��RΪCu���������������������ܶѻ���Cuԭ�Ӵ��ڶ��������ģ���Խ����ϵ�ԭ�����У�Cuԭ��֮��Ϊa pm�����ⳤ=a��10-10 cm��2��$\frac{\sqrt{2}}{2}$=$\sqrt{2}$a��10-10 cm�����ݾ�̯�����㾧����Cuԭ����Ŀ����ʾ�������������ٸ��ݦ�=$\frac{m}{V}$���㾧���ܶȣ�
��� �⣺X��Y��Z��M��W��R��Ԫ�����ڱ���1��36��Ԫ�أ���ԭ��������������YԪ�ػ�̬ԭ�ӵ�2P������ڰ����״̬��ԭ�Ӻ�������Ų�ʽΪ1s22s22p3����YΪNԪ�أ�X��Y��Z��λ��ͬһ���ڵ�����Ԫ�أ����ԭ��������֪XΪCԪ�ء�ZΪOԪ�أ�MΪԪ�����ڱ�1��36��Ԫ���е縺����С��Ԫ�أ���MΪK��WԪ�ػ�̬ԭ�ӵļ۵��ӹ���Ϊ3d74s2����WΪCo��Rλ�����ڱ���11�У�ԭ����������Co����RΪCu��
��1��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���Ԫ��2p�ܼ�����3������Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ��ʵ�һ�������ɴ�С��˳����N��O��C��
Y�������̬�⻯��ΪNH3��X�������̬�⻯��ΪCH4��NH3������ˮ����֮���γ����������������ˮ���Ӷ��Ǽ��Է��ӣ��������ܣ�ʹ������ˮ�е��ܽ��Զ���ڼ���ģ�
�ʴ�Ϊ��N��O��C��������ˮ����֮���γ����������������ˮ���Ӷ��Ǽ��Է��ӣ��������ܣ�
��2��RΪCu�����������Ϊ29��Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪʽΪ1s22s22p63s23p63d104s1��Z��M�γɻ�����M2Z2ΪK2O2������ʽΪ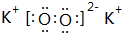 ��
��
�ʴ�Ϊ��1s22s22p63s23p63d104s1��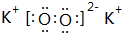 ��
��
��3��X��Z��W����Ԫ�ؿ����γ��ٺ�ɫ�������Ĺ��������Co2��CO��8������������ṩ�չ������Co���ṩ�¶Ե��ӵ���CO��
�ʴ�Ϊ��Co��CO��
��4���Ӹ����ġ�������Ľṹͼ��֪��Cԭ���γ�4������Nԭ���γ�3�������û�����Ļ�ѧʽΪC3N4�����仯ѧ��Ϊ���ۼ������Ӳ�ȴ��֪��Ϊԭ�Ӿ��壬̼ԭ���γ�4����������Ϊsp3�ӻ�������Nԭ���γ�3������������1�Թ¶Ե��ӣ��۲���Ӷ���Ϊ4�����Nԭ��Ҳ��sp3�ӻ���
�ʴ�Ϊ��C3N4��ԭ�Ӿ��壻sp3��
��5��RΪCu���������������������ܶѻ���Cuԭ��λ��Ϊ ����Խ����ϵ�ԭ�����У�Cuԭ��֮��Ϊa pm�����ⳤ=a��10-10 cm��2��$\frac{\sqrt{2}}{2}$=$\sqrt{2}$a��10-10 cm��������Cuԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Cu�����ԭ������ΪM����ʾ�������Ϊ4��$\frac{M}{{N}_{A}}$g�����ܶ�Ϊ4��$\frac{M}{{N}_{A}}$g�£�$\sqrt{2}$a��10-10 cm��3=$\frac{\sqrt{2}M��1{0}^{30}}{{a}^{3}{N}_{A}}$g•cm-3��
����Խ����ϵ�ԭ�����У�Cuԭ��֮��Ϊa pm�����ⳤ=a��10-10 cm��2��$\frac{\sqrt{2}}{2}$=$\sqrt{2}$a��10-10 cm��������Cuԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Cu�����ԭ������ΪM����ʾ�������Ϊ4��$\frac{M}{{N}_{A}}$g�����ܶ�Ϊ4��$\frac{M}{{N}_{A}}$g�£�$\sqrt{2}$a��10-10 cm��3=$\frac{\sqrt{2}M��1{0}^{30}}{{a}^{3}{N}_{A}}$g•cm-3��
�ʴ�Ϊ�� ��$\frac{\sqrt{2}M��1{0}^{30}}{{a}^{3}{N}_{A}}$��
��$\frac{\sqrt{2}M��1{0}^{30}}{{a}^{3}{N}_{A}}$��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų���Ԫ�������ɡ������ܡ�����ʽ�������������������ȣ�ע��ͬ��������Ԫ���е�һ�������쳣��������վ�̯�����о����йؼ��㣬�Ƕ�ѧ���ۺ������Ŀ��飮

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�| A�� | ��SO2ͨ����ˮ��֤��SO2����Ư���� | |
| B�� | ����м����ϡHNO3��֤��Fe��H2���� | |
| C�� | ������ʯ��ˮ����ij��Һ֤�����д���CO32- | |
| D�� | ����ϩͨ��KMnO4������Һ֤����ϩ���л�ԭ�� |
| A�� | ����ԭ�Ӻ�������Ų����ص㣬Zn�����ڱ�������ds��Ԫ�� | |
| B�� | P4��CH4�������������η����Ҽ��Ƕ�Ϊ109��28�� | |
| C�� | NH3������Nԭ�Ӻ�H2O������Oԭ�ӵ��ӻ����Ͳ���ͬ | |
| D�� | ԭ�Ӽ�ֻͨ�����ۼ����γɵ���ά��״�ṹ�ľ���һ�����иߵ��ۡ��е㼰Ӳ�� |
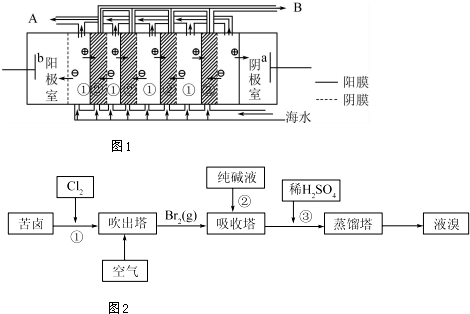
 ����������ͭ��ˮ�Ĺ���������м���Ӧ��DZ�ܣ��Ǽ��߿���ǰ������ɫ�����������Ŀǰ��Ҫ�ĺϳɷ����е�ⷨ�����¹��෨�ȣ�
����������ͭ��ˮ�Ĺ���������м���Ӧ��DZ�ܣ��Ǽ��߿���ǰ������ɫ�����������Ŀǰ��Ҫ�ĺϳɷ����е�ⷨ�����¹��෨�ȣ���1�����о����������������ɹ��Ƶ���Cu2O �������У�װ����ͼ���õ�ص�������Ӧ����ʽΪ2Cu-2e-+2OH-=Cu2O+H2O���ӽ���ĤΪ�����������������ӽ���Ĥ��ͭ��Ӧ���ӵ�Դ��������
��2���ڸ������ü��齫��״CuO ��ԭҲ���Ƶ�Cu2O��
��֪����2Cu��s��+$\frac{1}{2}$O2��g��=Cu2O��s������H=-169kJ•mol-1
��CH4��g��+2O2��g��=CO2��g��+2H2O��g������H=-846.3kJ•mol-1
��Cu��s��+$\frac{1}{2}$O2��g��=CuO��s������H=-157kJ•mol-1
��÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�8CuO��s��+CH4��g��=4Cu2O��s��+CO2��g��+2H2O��g����H=-266.3kJ/mol��
��3������ͬ���ܱ������У��õ���������������Cu2O���ò�ͬ�����Ƶã��ֱ���д��ֽ�ˮ��ʵ�飺2H2O��g��$?_{Cu_{2}O}^{����}$2H2��g��+O2��g����H��0��ˮ����Ũ����ʱ��t�仯���±���ʾ��
| ��� |  | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
��ʵ���ǰ20min��ƽ����Ӧ���� v��O2��=3.5��10-5mol/��L•min��
�۱Ƚϲ�ͬ�����Ƶõ�Cu2O�Ĵ�Ч��Ӧѡ�âٺ͢���ʵ�飬ԭ���dz������⣬����������ͬ��
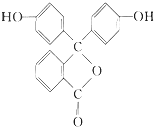
| A�� | ��̪���������ԣ������ڷ����� | |
| B�� | ��̪�ķ���ʽΪC19H12O4 | |
| C�� | 1mol��̪�����2molNaOH������Ӧ | |
| D�� | ��̪�ڼ����������ܹ�����ˮ�ⷴӦ�����ֺ�ɫ |
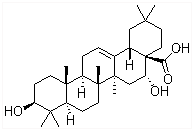 ����������̸β�Ѫ����ѪֹѪ���ܣ��ṹʽ��ͼ��ʾ�����й��ڴ������˵������ȷ���ǣ�������
����������̸β�Ѫ����ѪֹѪ���ܣ��ṹʽ��ͼ��ʾ�����й��ڴ������˵������ȷ���ǣ�������| A�� | ������������������к���ȩ�� | B�� | �ܺ�NaHCO3��Һ��Ӧ����CO2 | ||
| C�� | ��ʹ���Ը��������Һ��ɫ | D�� | �ܷ���ȡ�����������ӳɵȷ�Ӧ |
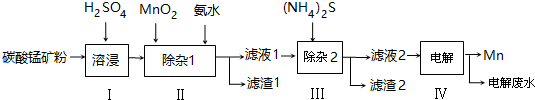
��֪25�棬�������ʵ��ܶȻ��������£�
| ���� | Mn��OH��2 | Co��OH��2 | Ni��OH��2 | MnS | CoS | NiS |
| Ksp | 2.1��10-13 | 3.0��10-16 | 5.0��10-16 | 1.0��10-11 | 5.0��10-22 | 1.0��10-22 |
��2��������У���Ӱ�ˮ������Һ��pHΪ5.0��6.0��������1����Ҫ�ɷ�ΪFe��OH��3���ѧʽ������֪MnO2������Ϊ����������õ��óɷ����漰�����ӷ���ʽΪ2Fe2++MnO2+4H+=2Fe3++Mn2++2H2OFe3++3NH3�qH2O=Fe��OH��3��+3NH4+��
��3��������У����ӣ�NH4��2S��Ũ�Ȳ��˹����ԭ��������NH4��2S��Ũ�ȹ�����MnS��������ɲ�Ʒ��ʧ��
��4����Һ2�У�c��Co2+����c��Ni2+��=5��1��
��5��������Ϊa�K��̼���̿��������̴�����õ�����Mn b kg����ÿһ����������ȫ������1Ϊ���������Ϊc kg����ԭ̼���̿���MnCO3����������Ϊ$\frac{��b-\frac{c}{107}��\frac{1}{2}��55����\frac{115}{55}}{a}$��100%�����ú�a��b��c��ʽ�ӱ�����軯��
 ���������ҹ�������������Ƶ��������������PM2.5ϸ���Ӱ�����NH4��2 SO4��NH4NO3���л����P�ﳾ�ȣ�ͨ���ⶨ������п���ؽ����ĺ�������֪��ͨ��Ⱦ��Ŀǰ���������������Ҫԭ��֮һ���ش��������⣺
���������ҹ�������������Ƶ��������������PM2.5ϸ���Ӱ�����NH4��2 SO4��NH4NO3���л����P�ﳾ�ȣ�ͨ���ⶨ������п���ؽ����ĺ�������֪��ͨ��Ⱦ��Ŀǰ���������������Ҫԭ��֮һ���ش��������⣺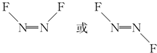 ��1molN2F2�����������Ҽ�����Ŀ��3NA��
��1molN2F2�����������Ҽ�����Ŀ��3NA��