��Ŀ����
��1��ʵ������Ҫ����220mL��1mol/L��ϡ����Ӧѡ�õ�����ƿ����ǣ� ��������ʱ������ƫ�ͣ�����õ���Һ�������ʵ���Ũ�� 1mol/L���������=������������
��2�������й�ʵ�������˵����ȷ����
A������25mL��ʽ�ζ�����ȡ20.00mLKMnO4��Һ
B����PH��ֽ�ⶨ��Һ��PHʱ������������ˮ��ʪ��ֽ
C������ʱ������ƿ��Һ���������ܳ����ݻ���
��Һ��Ҳ��������
D�������������в����гɷ�ĩ��ʹ����ˮ��Ӧ��ʵ�����ȫ
E����NaOH��Һϴ�Ӳ����ղ�˿���ٽ�����ɫ��Ӧ��
��2�������й�ʵ�������˵����ȷ����
A������25mL��ʽ�ζ�����ȡ20.00mLKMnO4��Һ
B����PH��ֽ�ⶨ��Һ��PHʱ������������ˮ��ʪ��ֽ
C������ʱ������ƿ��Һ���������ܳ����ݻ���
| 2 |
| 3 |
D�������������в����гɷ�ĩ��ʹ����ˮ��Ӧ��ʵ�����ȫ
E����NaOH��Һϴ�Ӳ����ղ�˿���ٽ�����ɫ��Ӧ��
���㣺����һ�����ʵ���Ũ�ȵ���Һ,��ѧʵ�鷽��������
ר�⣺ʵ��������
��������1��ʵ��������Ҫ����1mol/L��ϡ����220mL������û��220mL������ƿ��ʵ������ʱ��Ҫѡ��250mL����ƿ������c=
�����ж������nƫС��Vƫ����������ҺŨ��ƫ�ͣ�
��2��A��������ؾ��������ԣ���ʴ��Ƥ�ܣ�
B����pH��ֽ�ⶨδ֪��Һ��pHʱ���ò�����պȡ��������Һ���ڸ����pH��ֽ�ϣ������ɫ���Ա���ȷ��pH��������ˮʪ��pH��ֽ������ϡ���˴�����Һ��ʹ��Һ������Լ�����
C����ѹ����ʱ��������ƿ����ʢ��Һ�岻�ܳ������ݻ���
��Ҳ��������
����ʱ�����ܽ�Һ�����ɣ�
D���ƿ��ˮ��Ӧ��ʮ�־��ң���ĥ�ɷ�ĩ��ˮ��Ӧ̫���ң�
E����ɫ��Ӧʱ��ϡ����ϴ�Ӳ�˿��
| n |
| V |
��2��A��������ؾ��������ԣ���ʴ��Ƥ�ܣ�
B����pH��ֽ�ⶨδ֪��Һ��pHʱ���ò�����պȡ��������Һ���ڸ����pH��ֽ�ϣ������ɫ���Ա���ȷ��pH��������ˮʪ��pH��ֽ������ϡ���˴�����Һ��ʹ��Һ������Լ�����
C����ѹ����ʱ��������ƿ����ʢ��Һ�岻�ܳ������ݻ���
| 2 |
| 3 |
| 1 |
| 3 |
D���ƿ��ˮ��Ӧ��ʮ�־��ң���ĥ�ɷ�ĩ��ˮ��Ӧ̫���ң�
E����ɫ��Ӧʱ��ϡ����ϴ�Ӳ�˿��
���
�⣺��1������ʵ����û�й��Ϊ220mL������ƿ��������Һʱ��Ҫѡ��250mL������ƿ������ʵ�����Ƶ���ҺΪ��250mL 1mol/L��ϡ���
������ʱ������ƫ�ͣ�������Һ���ƫ������õ���Һ�������ʵ���Ũ��ƫС��
�ʴ�Ϊ��250mL������
��2��A��������ؾ��������ԣ���ʴ��Ƥ�ܣ�Ӧ����ʽ�ζ��ܣ���A����
B��ˮʪ��pH��ֽ��ϡ���˴�����Һ��ʹ��Һ������Լ��������ⶨ����������Һ�����ʹ�ⶨ���������ⶨ���Ǽ�����Һ�����ʹ�ⶨ�����С�����ⶨ����������Һ�����ʹ�ⶨ������䣬��B����
C������ʱ������ƿ��Һ���������ܳ����ݻ���
��Һ��Ҳ�������ɣ���C��ȷ��
D���ƿ��ˮ��Ӧ��ʮ�־��ң���ĥ�ɷ�ĩ��ˮ��Ӧ̫���ң���D����
E��������ϴ�Ӳ����ղ�˿���ٽ�����ɫ��Ӧ����E����
��ѡ��C��
������ʱ������ƫ�ͣ�������Һ���ƫ������õ���Һ�������ʵ���Ũ��ƫС��
�ʴ�Ϊ��250mL������
��2��A��������ؾ��������ԣ���ʴ��Ƥ�ܣ�Ӧ����ʽ�ζ��ܣ���A����
B��ˮʪ��pH��ֽ��ϡ���˴�����Һ��ʹ��Һ������Լ��������ⶨ����������Һ�����ʹ�ⶨ���������ⶨ���Ǽ�����Һ�����ʹ�ⶨ�����С�����ⶨ����������Һ�����ʹ�ⶨ������䣬��B����
C������ʱ������ƿ��Һ���������ܳ����ݻ���
| 2 |
| 3 |
D���ƿ��ˮ��Ӧ��ʮ�־��ң���ĥ�ɷ�ĩ��ˮ��Ӧ̫���ң���D����
E��������ϴ�Ӳ����ղ�˿���ٽ�����ɫ��Ӧ����E����
��ѡ��C��
���������⿼���˳�����ʵ�������һ�����ʵ���Ũ����Һ�����Ƶ�֪ʶ����Ŀ�ѶȲ���ע������220mL��Һ��ʵ�����Ƶ���Һ�ݻ���250mL��

��ϰ��ϵ�д�
�����Ŀ
2A��g���T2B��g��+C��g����H��0����ƽ��ʱ��ҪʹV�����ͣ�Ӧ��ȡ��������
| A����ѹ | B����ѹ |
| C������ | D��������� |
���н���ʵ������ķ�Ӧ����ʽ��ȷ���ǣ�������
| A����Cu��OH��2����Һ�еμ�Na2S��Һ����ɫ�������ɫCu(OH)2(s)+S2-?CuS��s��+2OH- |
| B����H2O2��Һ�У��μ�FeC13��Һ�������ݣ�2H2O2+2C1-=2H2O+O2��+C12�� |
| C������Ӵ���ͭƬ��пƬ����ϡ�����У�ͭƬ���������ݲ�����Cu+2H+=Cu2++H2�� |
| D����CH3COONa��Һ�У��μӷ�̪��죺CH3COO-+H2O=CH3COOH+OH- |
������ʵ��֤��һˮ�ϰ���������ʵ��ǣ�������
| A��0.1mol/L�İ�ˮ��ʹ��̪��Һ��� |
| B��0.1mol/L���Ȼ����Һ��pHԼΪ5 |
| C������ͬ�����£���ˮ��Һ�ĵ����Ա�ǿ����Һ�� |
| D����������ֽ� |
 �����ʵ���Ũ����ȵ�CuSO4��Һ��NaCl��Һ�������Ϻ���ʯī�缫���е�⣬�������У���Һ��pH��ʱ��t�仯��������ͼ��ʾ��������˵���в���ȷ���ǣ�������
�����ʵ���Ũ����ȵ�CuSO4��Һ��NaCl��Һ�������Ϻ���ʯī�缫���е�⣬�������У���Һ��pH��ʱ��t�仯��������ͼ��ʾ��������˵���в���ȷ���ǣ�������| A��A��pHС��7����ΪCu2+ˮ��ʹ��Һ������ |
| B��BC������������Cl2 |
| C�����������������Ȳ���Cl2�������O2 |
| D��CD�ε���������ˮ |
 ijʵ��С���ȡ���²���ⶨij������Ʒ�Ĵ��ȣ�
ijʵ��С���ȡ���²���ⶨij������Ʒ�Ĵ��ȣ�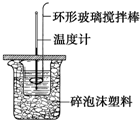 ������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽�����£�