��Ŀ����
�����ס��غųƷ��ϵġ���Ҫ�ء�����ѧ���ϵ�ʩ�ã�ʹũҵ�������գ�����Ч�����������ǣ���ѧ���ϵ���ʩ����Ҳ�Ի��������������Ӱ�죮��������֪ʶ����Ϣ�ش��������⣺
��1����ͼ��ijƷ�ƻ��ʰ�װ���еIJ�������

��д����Ϊԭ����ȡ�û��ʵĸ�����ѧ����ʽ ��
�ڹ��ڸõ��ʵĴ��ú�ʩ����ȷ�ķ����� ��
A�����ľ�һ��ʩ��
B�������飬���������û�
C�����þ�ʻ��ʩ��
D���ھ����Ź�ˮ�ĵ�����ʩ�÷�Ч���
�����û��ʵ������в�����Ԫ�أ���û��ʵĴ����� ��
��2���ס��ҡ����������ʳ������������õ�ԭ�ϲ�ͬ��������������ͬ��

�ټ׳��Խ�̿��ˮΪԭ�ϣ����ҳ�����Ȼ����ˮΪԭ�ϣ��۱�����ʯ���ͣ���Ҫ�ɷ�ΪC5H12����ˮΪԭ�ϣ�����ҵ�йع涨������ԭ�����Ƶõ�ԭ����H2��CO2�����ʵ���֮�ȣ�����ӽ��ϳ����ص�ԭ����NH3�������H2�����ʵ�������CO2�����ʵ���֮�ȣ����ԭ�ϵ���������ߣ��ݴ��жϼס��ҡ������������ĸ�������ԭ�ϵ���������ߣ� ��
��3����������ˮ�����͡��ೱ��ʱ�з�������ר�ҷ���������ˮ�帻Ӫ������ɵģ��Է���ˮ�帻Ӫ������ԭ��
��
��1����ͼ��ijƷ�ƻ��ʰ�װ���еIJ�������

��д����Ϊԭ����ȡ�û��ʵĸ�����ѧ����ʽ
�ڹ��ڸõ��ʵĴ��ú�ʩ����ȷ�ķ�����
A�����ľ�һ��ʩ��
B�������飬���������û�
C�����þ�ʻ��ʩ��
D���ھ����Ź�ˮ�ĵ�����ʩ�÷�Ч���
�����û��ʵ������в�����Ԫ�أ���û��ʵĴ�����
��2���ס��ҡ����������ʳ������������õ�ԭ�ϲ�ͬ��������������ͬ��

�ټ׳��Խ�̿��ˮΪԭ�ϣ����ҳ�����Ȼ����ˮΪԭ�ϣ��۱�����ʯ���ͣ���Ҫ�ɷ�ΪC5H12����ˮΪԭ�ϣ�����ҵ�йع涨������ԭ�����Ƶõ�ԭ����H2��CO2�����ʵ���֮�ȣ�����ӽ��ϳ����ص�ԭ����NH3�������H2�����ʵ�������CO2�����ʵ���֮�ȣ����ԭ�ϵ���������ߣ��ݴ��жϼס��ҡ������������ĸ�������ԭ�ϵ���������ߣ�
��3����������ˮ�����͡��ೱ��ʱ�з�������ר�ҷ���������ˮ�帻Ӫ������ɵģ��Է���ˮ�帻Ӫ������ԭ��
���㣺ԭ������Դ�ĺ�������
ר�⣺Ԫ�ؼ��仯����
��������1���ٰ�������������NO��NO��������Ӧ����NO2��NO2��ˮ��Ӧ�������ᣬ����Ͱ�����Ӧ��������泥�
���̬���ʲ������ľ�һ��ʹ�ã��������NH3�ݳ���NH4NO3��飬����������������ը��NH4NO3��ˮ�⣻
��NH4NO3�ĺ�����Ϊ35%����=
=98%��
��2�����ݼס��ҡ�������Ҫ��Ӧ�ֱ�ΪC+H2O��g��
CO+H2��CH4+H2O
CO+3H2��C5H12+5H2O
5CO+11H2����������COת��CO2���䷴ӦʽCO+H2O
CO2+H2������ס��ҡ�������H2��CO2�����ʵ�����Ϊ��
��3��������Ȼˮ�������ڹ���Ӫ�����ʣ���Ҫ��ָ�����ȣ������룬�������ˮ�����ֲ���쳣��ֳ�������������������ˮ�帻Ӫ�����Լ������ʺ��е�Ԫ�ط�����
���̬���ʲ������ľ�һ��ʹ�ã��������NH3�ݳ���NH4NO3��飬����������������ը��NH4NO3��ˮ�⣻
��NH4NO3�ĺ�����Ϊ35%����=
| 34.3% |
| 35% |
��2�����ݼס��ҡ�������Ҫ��Ӧ�ֱ�ΪC+H2O��g��
| ||
| ||
| ||
| ||
��3��������Ȼˮ�������ڹ���Ӫ�����ʣ���Ҫ��ָ�����ȣ������룬�������ˮ�����ֲ���쳣��ֳ�������������������ˮ�帻Ӫ�����Լ������ʺ��е�Ԫ�ط�����
���
�⣺��1���ٰ�������������NO��NO��������Ӧ����NO2��NO2��ˮ��Ӧ�������ᣬ����Ͱ�����Ӧ��������泥���ѧ����ʽΪ��4NH3+5O2
4NO+6H2O��
2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3��
���̬���ʲ������ľ�һ��ʹ�ã��������NH3�ݳ���NH4NO3��飬����������������ը��NH4NO3��ˮ��Ϊһˮ�ϰ�������N����ʧ��
�ʴ�Ϊ��C��
�۸û��ʵ������в�����Ԫ�أ�NH4NO3�ĺ�����Ϊ35%����=
=98%��
�ʴ�Ϊ��98%��
��2���ס��ҡ�������Ҫ��Ӧ�ֱ�Ϊ
C+H2O��g��
CO+H2��CH4+H2O
CO+3H2��C5H12+5H2O
5CO+11H2����������COת��CO2���䷴ӦʽCO+H2O
CO2+H2���ʼס��ҡ�������H2��CO2�����ʵ�����Ϊ��
�ף�
=2��1���ң�
=4��1��
����
=16��5��
���ϳ����ص�
=3��1ʱ������������ߣ�
�ʴ�Ϊ������
��3���������и��ֺ��������л���ķ���ˮ����ˮ�У���ʹˮ��Ӫ��������������ˮ���������ೱ����
�ʴ�Ϊ����N��P���ʵĴ���ʩ���Լ�������ˮ�Ĵ����ŷţ�����������ˮ������ʩ���ؽ�����ˮ�帻Ӫ��������������ˮ���������ೱ����
| ||
2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��NH3+HNO3�TNH4NO3��
�ʴ�Ϊ��4NH3+5O2
| ||
���̬���ʲ������ľ�һ��ʹ�ã��������NH3�ݳ���NH4NO3��飬����������������ը��NH4NO3��ˮ��Ϊһˮ�ϰ�������N����ʧ��
�ʴ�Ϊ��C��
�۸û��ʵ������в�����Ԫ�أ�NH4NO3�ĺ�����Ϊ35%����=
| 34.3% |
| 35% |
�ʴ�Ϊ��98%��
��2���ס��ҡ�������Ҫ��Ӧ�ֱ�Ϊ
C+H2O��g��
| ||
| ||
| ||
| ||
�ף�
| n(H2) |
| n(CO2) |
| n(H2) |
| n(CO2) |
����
| n(H2) |
| n(CO2) |
���ϳ����ص�
| n(H2) |
| n(CO2) |
�ʴ�Ϊ������
��3���������и��ֺ��������л���ķ���ˮ����ˮ�У���ʹˮ��Ӫ��������������ˮ���������ೱ����
�ʴ�Ϊ����N��P���ʵĴ���ʩ���Լ�������ˮ�Ĵ����ŷţ�����������ˮ������ʩ���ؽ�����ˮ�帻Ӫ��������������ˮ���������ೱ����
���������⿼����ԭ������Դ�ĺ������ã������ڻ�ѧ��Ӧ���̵ķ����ͼ��㡢������������Ŀ�Ѷ�һ�㣮

��ϰ��ϵ�д�
�����Ŀ
�����йػ�ѧ���뾧��ṹ��������ȷ���ǣ�������
| A��ˮ�����ɱ��ۻ�ʱ�˷����Ӽ���������������ͬ |
| B��12g���ʯ�У���C-C���ۼ�����Ϊ4mol |
| C�����Ӿ������ۻ�ʱ�����Ӽ����ƻ��������Ӿ����ۻ�ʱ����ѧ�������ƻ� |
| D���۵��ɸߵ��͵�˳���ǣ�����裾̼���裾���ʯ |
���и��������뾶������ԭ������ǿ��˳�����е��ǣ�������
| A��Na��K��Rb |
| B��F��Cl��Br |
| C��Mg2+��Al3+��Zn2+ |
| D��Cl-��Br-��I- |
NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A��4.6g���ΪC2H6O���л������C-H����Ŀһ��Ϊ0.6NA |
| B��12g���ʯ�к��еĹ��ۼ���Ϊ2NA |
| C��0.1mol�İ��ף�P4��������������ĦҼ�����Ϊ0.4NA |
D��0.5mol�ۻƣ�As4S4�ṹ��ͼ������NA��S-S��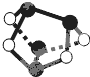 |
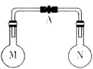 ����ʱ�������ݻ���ͬ����ƿ�зֱ�ʢ��M��N�������壨ͬ��ͬѹ����ȡ�µ��ɼ�A��ʹ����ƿ�ڵ�����Ӵ�����ͼ���������ڵ�ѹǿ�ɴ�С��˳���ǣ�������
����ʱ�������ݻ���ͬ����ƿ�зֱ�ʢ��M��N�������壨ͬ��ͬѹ����ȡ�µ��ɼ�A��ʹ����ƿ�ڵ�����Ӵ�����ͼ���������ڵ�ѹǿ�ɴ�С��˳���ǣ�������