��Ŀ����
A��B��C��D��E�����ֳ��������Σ��������ӿ�����K+��NH4+��Cu2+��Ba2+��Al3+��Ag+��Fe3+�������ӿ�����Cl-��NO3-��SO42-��CO32-����֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ����ɫ������ɫ�ܲ�������
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԣ�
�����������ε���Һ�м���Ba��NO3��2��Һ��ֻ��A��C����Һ������������
�����������ε���Һ�м��백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ����������������ᆳ�����ڼ����л����е�ȩ����
�ް�A����Һ�ֱ�ӵ�B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��ش���������
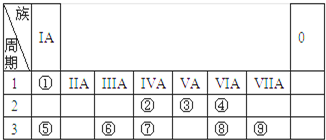
��1���������У�һ��û�е��������� ���������������У����Ӱ뾶��С���� ����Ԫ����Ԫ�����ڱ��е�λ��Ϊ
��2��D������Ϊ ��D��Һ�Լ��Ե�ԭ���ǣ������ӷ���ʽ��ʾ�� ��
��3��E�Ͱ�ˮ��Ӧ�����ӷ���ʽ�� ��
��4������B�������ӵ�ʵ�鷽���� ��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ����ɫ������ɫ�ܲ�������
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԣ�
�����������ε���Һ�м���Ba��NO3��2��Һ��ֻ��A��C����Һ������������
�����������ε���Һ�м��백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ����������������ᆳ�����ڼ����л����е�ȩ����
�ް�A����Һ�ֱ�ӵ�B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��ش���������
��1���������У�һ��û�е���������
��2��D������Ϊ
��3��E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��
��4������B�������ӵ�ʵ�鷽����
���㣺���������ӵļ���,���������ӵļ���
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
�������������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ����û��Cu2+��Fe3+��
��D����ɫ��Ӧ����ɫ����D����K+��
��A����Һ�����ԣ�B��C��E����Һ���������У�NH4+��Al3+��Ag+��D����Һ�ʼ��ԣ���D�к���CO32-�����������ӿ�֪DΪK2CO3��
�������������ε���Һ�зֱ����Ba��NO3��2��Һ��ֻ��A��C����Һ��������������A��C��û��CO32-��SO42-��
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��˵��C��ΪAg+����E����Al3+������CΪAgNO3��
�ް�A��Һ�����Էֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�������AΪBaCl2�������Ϸ�����֪E�к���Al3+��B�к���NH4+����BaCl2�������ɲ�����ϡ����ij�������B��E�к���SO42-������B��EΪ��NH4��2SO4��Al2��SO4��3�������Ŀ������
��D����ɫ��Ӧ����ɫ����D����K+��
��A����Һ�����ԣ�B��C��E����Һ���������У�NH4+��Al3+��Ag+��D����Һ�ʼ��ԣ���D�к���CO32-�����������ӿ�֪DΪK2CO3��
�������������ε���Һ�зֱ����Ba��NO3��2��Һ��ֻ��A��C����Һ��������������A��C��û��CO32-��SO42-��
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��˵��C��ΪAg+����E����Al3+������CΪAgNO3��
�ް�A��Һ�����Էֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�������AΪBaCl2�������Ϸ�����֪E�к���Al3+��B�к���NH4+����BaCl2�������ɲ�����ϡ����ij�������B��E�к���SO42-������B��EΪ��NH4��2SO4��Al2��SO4��3�������Ŀ������
���
�⣺�����ֵ�ˮ��Һ��Ϊ��ɫ����һ��û����ɫ�����ӣ�Cu2+��Fe3+��
��D����ɫ��Ӧ����ɫ����D����K+��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�����NH4+��Al3+��Ag+��D����Һ�ʼ�����D�к���CO32-����Ϣ������ӿ�֪DΪK2CO3��
�������������ε���Һ�зֱ����Ba��NO3��2��Һ��ֻ��A��C����Һ��������������A��C��û��SO42-��
�������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��˵��C��ΪAg+����E����Al3+������CΪAgNO3��
�ް�A��Һ�����Էֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�������AΪBaCl2�������Ϸ�����֪E�к���Al3+��B�к���NH4+����BaCl2�������ɲ�����ϡ����ij�������B��E�к���SO42-������B��EΪ��NH4��2SO4��Al2��SO4��3��
��1���������У�һ��û�е���������Cu2+��Fe3+��K+��Cu2+��Ba2+��Al3+��Ag+��Fe3+������K+��Cu2+��Fe3+����3����ӣ�Ba2+��5����ӣ�Ag+��4����ӣ�Al3+��2����ӣ������Ӱ뾶��С����Al3+����Ԫ�����ڱ��е�λ��Ϊ����3���ڣ���A�壬
�ʴ�Ϊ��Cu2+��Fe3+��Al3+����3���ڣ���A�壻
��2��D�Ļ�ѧʽΪK2CO3������Ϊ̼��أ�K2CO3��Һ�Լ��Ե�ԭ���ǣ�CO32-+H2O?HCO3-+OH-��
�ʴ�Ϊ��̼��أ�CO32-+H2O?HCO3-+OH-��
��3��Al2��SO4��3�Ͱ�ˮ��Ӧ�����ӷ���ʽΪ��Al3++3NH3?H2O�TAl��OH��3��+3NH4+��
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3��+3NH4+��
��4��BΪ��NH4��2SO4�����飨NH4��2SO4��������������笠����ӵķ���Ϊ��ȡ������NH4��2SO4���Թ��У��μ�����NaOH��Һ�����Թܿڸ�����һ��ʪ��ĺ�ɫʯ����ֽ�����ȣ�����ֽ������˵��B��������ΪNH4+��
�ʴ�Ϊ��ȡ��������Һ��������������������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ���������壮
��D����ɫ��Ӧ����ɫ����D����K+��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�����NH4+��Al3+��Ag+��D����Һ�ʼ�����D�к���CO32-����Ϣ������ӿ�֪DΪK2CO3��
�������������ε���Һ�зֱ����Ba��NO3��2��Һ��ֻ��A��C����Һ��������������A��C��û��SO42-��
�������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��˵��C��ΪAg+����E����Al3+������CΪAgNO3��
�ް�A��Һ�����Էֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�������AΪBaCl2�������Ϸ�����֪E�к���Al3+��B�к���NH4+����BaCl2�������ɲ�����ϡ����ij�������B��E�к���SO42-������B��EΪ��NH4��2SO4��Al2��SO4��3��
��1���������У�һ��û�е���������Cu2+��Fe3+��K+��Cu2+��Ba2+��Al3+��Ag+��Fe3+������K+��Cu2+��Fe3+����3����ӣ�Ba2+��5����ӣ�Ag+��4����ӣ�Al3+��2����ӣ������Ӱ뾶��С����Al3+����Ԫ�����ڱ��е�λ��Ϊ����3���ڣ���A�壬
�ʴ�Ϊ��Cu2+��Fe3+��Al3+����3���ڣ���A�壻
��2��D�Ļ�ѧʽΪK2CO3������Ϊ̼��أ�K2CO3��Һ�Լ��Ե�ԭ���ǣ�CO32-+H2O?HCO3-+OH-��
�ʴ�Ϊ��̼��أ�CO32-+H2O?HCO3-+OH-��
��3��Al2��SO4��3�Ͱ�ˮ��Ӧ�����ӷ���ʽΪ��Al3++3NH3?H2O�TAl��OH��3��+3NH4+��
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3��+3NH4+��
��4��BΪ��NH4��2SO4�����飨NH4��2SO4��������������笠����ӵķ���Ϊ��ȡ������NH4��2SO4���Թ��У��μ�����NaOH��Һ�����Թܿڸ�����һ��ʪ��ĺ�ɫʯ����ֽ�����ȣ�����ֽ������˵��B��������ΪNH4+��
�ʴ�Ϊ��ȡ��������Һ��������������������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ���������壮
������������Ҫ�������ӹ�������ӷ�Ӧ����Ŀ�Ѷ��еȣ�ע�����ճ������ӵļ��鷽�����ڽ������ʱ�����Ƚ������������������Ƴ���Ȼ�����Ƴ��������Ƶ�ʣ������ʣ���������֤���ɣ�

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ
���㻯����M�Ľṹ��ʽΪ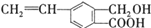 �������л���M��˵����ȷ���ǣ�������
�������л���M��˵����ȷ���ǣ�������
 �������л���M��˵����ȷ���ǣ�������
�������л���M��˵����ȷ���ǣ�������| A���л���M�ķ���ʽΪC10H12O3 |
| B��1mol Na2CO3���������1mol���M |
| C��1mol M�����������Ʒ�Ӧ����22.4L���� |
| D���л���M�ܷ���ȡ���������ͼӳɷ�Ӧ |
��һ������Fe��FeO��Fe2O3�Ļ�����м���120mL5mol/L��ϡ���ᣬǡ��ʹ�������ȫ�ܽ⣬�ų�2.24L NO����״��������������Һ�м���KSCN��Һ����ɫ���֣����������������ڼ����»�ԭ��ͬ�����Ļ����ܵõ��������ʵ���Ϊ��������
| A��0.24 mol |
| B��0.21 mol |
| C��0.25 mol |
| D��0.14 mol |
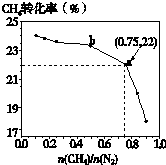 ��֪��3CH4��g��+2N2��g��
��֪��3CH4��g��+2N2��g��| 700�� |
| ���� |
| n(CH4) |
| n(N2) |
A��
| ||
B��
| ||
| C��b���Ӧ��ƽ�ⳣ����a��Ĵ� | ||
| D��a���Ӧ��NH3���������ԼΪ26% |