��Ŀ����
��һ�������£�N2O�ֽ�IJ���ʵ���������£�������
��ͼ����ȷ��ʾ�÷�Ӧ�й��������仯���ɵ��ǣ�������
��ע��ͼ�а�˥��ָ��һŨ��N2O����һ��ʱ�������Ӧʱ�䣬c1��c2����ʾN2O��ʼŨ����c1��c2��
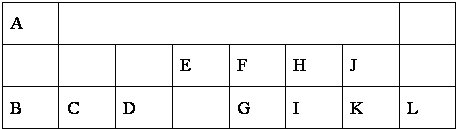
| ��Ӧʱ��/min | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
| c��N2O��/mol?L-1 | 0.100 | 0.090 | 0.080 | 0.070 | 0.060 | 0.050 | 0.040 | 0.030 | 0.020 | 0.010 | 0.000 |
��ע��ͼ�а�˥��ָ��һŨ��N2O����һ��ʱ�������Ӧʱ�䣬c1��c2����ʾN2O��ʼŨ����c1��c2��
A��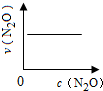 |
B��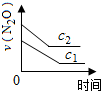 |
C��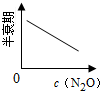 |
D��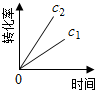 |
���㣺ת�������¶ȡ�ѹǿ�ı仯����,����ٷֺ������¶ȡ�ѹǿ�仯����
ר�⣺��ѧƽ��ר��
���������ݱ����е����ݺ�����ͼ���Ӱ�컯ѧ��Ӧ���ʺͻ�ѧƽ������ؽ����жϵó���ȷ���ۣ�
���
�⣺A���ɱ���֪��ÿ��10min��c��N2O���ı仯����ȣ��ʵ�λʱ����c��N2O���ı仯���Ƕ�ֵ����N2O�ķֽ������Ƕ�ֵ����A��ȷ��
B���ɱ���֪��ÿ��10min��c��N2O���ı仯����ȣ��ʵ�λʱ����c��N2O���ı仯��N2O����ʼŨ���أ�����N2O��ȫ�ֽ⣬��B����
C.0-50min��c��N2O����0.1��Ϊ0.05����0.1mol?L-1N2O�İ�˥��Ϊ50min��20-60min��c��N2O����0.08��Ϊ0.04����0.08mol?L-1N2O�İ�˥��Ϊ40min��������Ũ�ȵļ�С����˥��Ҳ�ڼ�С����C����
D���ɱ���֪��ÿ��10min��c��N2O���ı仯����ȣ���N2O����ʼŨ��ԽС����λʱ���ڵ�ת����Խ������N2O��ȫ�ֽ⣬��D����
��ѡA��
B���ɱ���֪��ÿ��10min��c��N2O���ı仯����ȣ��ʵ�λʱ����c��N2O���ı仯��N2O����ʼŨ���أ�����N2O��ȫ�ֽ⣬��B����
C.0-50min��c��N2O����0.1��Ϊ0.05����0.1mol?L-1N2O�İ�˥��Ϊ50min��20-60min��c��N2O����0.08��Ϊ0.04����0.08mol?L-1N2O�İ�˥��Ϊ40min��������Ũ�ȵļ�С����˥��Ҳ�ڼ�С����C����
D���ɱ���֪��ÿ��10min��c��N2O���ı仯����ȣ���N2O����ʼŨ��ԽС����λʱ���ڵ�ת����Խ������N2O��ȫ�ֽ⣬��D����
��ѡA��
���������⿼��ƽ��ͼ��������Ѷ��еȣ���������ͼ����ͼ����ӱ����Ϣ���������˰�˥�ڵ�ע�ͣ��������Ѷȣ�������ѧ�����⣮

��ϰ��ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A����˿����������ø��������ˮ�����ɵõ���-������ |
| B��ijЩ���������γɵķ���ɸ����������״��Ѩ��ͨ������������������ӽ���������������������� |
| C������������۽�һ�����������Ƕ�������ʶ�����Խ���һЩ��ˮ��Һ�н��е���Ӧ������ |
| D������ɨ������������Ӧ��STM������ʵ�ֶ�ԭ�ӻ���ӵIJ��� |
�����£���1mol��CuSO4?5H2O��s������ˮ��ʹ��Һ�¶Ƚ��ͣ���ЧӦΪ��H1����1mol��CuSO4��s������ˮ��ʹ��Һ�¶����ߣ���ЧӦΪ��H2��CuSO4?5H2O���ȷֽ�Ļ�ѧ����ʽΪCuSO4?5H2O��s��
CuSO4��s��+5H2O��l������ЧӦΪ��H3���������ж���ȷ���ǣ�������
| ||
| A����H2����H3 |
| B����H1����H3 |
| C����H1+��H3=��H2 |
| D����H1+��H2����H3 |
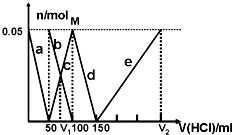 ijNa2CO3��NaAlO2�Ļ����Һ����μ���1mol?L-1�����ᣬ�����Һ�е�CO32-��HCO3-��AlO2-��Al3+���ӵ����ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵������ȷ���ǣ�������
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1mol?L-1�����ᣬ�����Һ�е�CO32-��HCO3-��AlO2-��Al3+���ӵ����ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵������ȷ���ǣ�������| A��ԭ�����Һ�е�CO32-��AlO2-�����ʵ���֮��Ϊ1��2 |
| B��V1��V2=1��4 |
| C��M��ʱ���ɵ�CO2Ϊ0mol |
| D��a���߱�ʾ�����ӷ���ʽΪ��AlO2-+H++H2O�TAl��OH��3�� |
Fe��Fe2O3�Ļ�������200mL 5mol?L-1�����ᣬǡ����ȫ�ܽ⣬�������м���KSCN��Һ��δ��Ѫ��ɫ����������Һ��Fe2+�����ʵ���Ũ��Ϊ�����跴Ӧ����Һ�����Ϊ200mL����������
| A��lmol?L-1 |
| B��2mol?L-1 |
| C��2.5mol?L-1 |
| D��5mol?L-1 |