��Ŀ����
��1�������������ֱ�����������ȫȼ�գ�
�����������ʵ�����ȣ����������������� ��
��������������ȣ����������������� ��
������Ӧǰ��150�棩��������ʵ����������仯������������� ��
A��C2H6 B��C2H4 C��C4H10 D��C5H10
��2�����и��黯�����У����۶�����ʲô������ϣ���ȫȼ��ʱ��
�������������䣬��O2��������� ������ˮ������������� ��
�����ܵ����ʵ������䣬���O2��������� ������ˮ������������� ������CO2������������� ��
A��C2H4��C3H6 B��HCHO��CH3COOH C��CH4��CH3COOH
D��CH2=CH2��CH3CH2OH E��C8H10��C4H7��OH��3��
�����������ʵ�����ȣ�����������������
��������������ȣ�����������������
������Ӧǰ��150�棩��������ʵ����������仯�������������
A��C2H6 B��C2H4 C��C4H10 D��C5H10
��2�����и��黯�����У����۶�����ʲô������ϣ���ȫȼ��ʱ��
�������������䣬��O2���������
�����ܵ����ʵ������䣬���O2���������
A��C2H4��C3H6 B��HCHO��CH3COOH C��CH4��CH3COOH
D��CH2=CH2��CH3CH2OH E��C8H10��C4H7��OH��3��
���㣺��ѧ����ʽ���йؼ���,�йػ���ﷴӦ�ļ���
ר�⣺�������������ȼ�չ���
��������1������ͬ���ʵ�������CxHy��ȫȼ�գ�������ȡ���ڣ�x+
������x+
��ֵԽ������Խ�ࣻ
����ͬ������CxHyȼ�պ��������ɣ������������Խ����ȫȼ�պ���Խ�࣮��
Խ������Խ��
������Ӧǰ��150�棩��������ʵ����������仯������CxHy��Hԭ����ĿΪ4��
��2���������������䣬��O2�����䣬���ʽ��ͬ�������㣻����ˮ���������䣬�������HԪ������������ȣ�
�����ܵ����ʵ������䣬���O2�����䣬��������x+
-
����ȣ�����CO2���������䣬�������Cԭ����Ŀ��ȣ�����ˮ���������䣬�������HԪ������ͬ��
| y |
| 4 |
| y |
| 4 |
����ͬ������CxHyȼ�պ��������ɣ������������Խ����ȫȼ�պ���Խ�࣮��
| y |
| x |
������Ӧǰ��150�棩��������ʵ����������仯������CxHy��Hԭ����ĿΪ4��
��2���������������䣬��O2�����䣬���ʽ��ͬ�������㣻����ˮ���������䣬�������HԪ������������ȣ�
�����ܵ����ʵ������䣬���O2�����䣬��������x+
| y |
| 4 |
| z |
| 2 |
���
�⣺��1��������CxHy�����ʵ�����ȣ�������ȡ���ڣ�x+
������x+
��Խ������Խ��C5H10��Cԭ�ӡ�Hԭ����Ŀ���C5H10�ĺ��������
�ʴ�Ϊ��D��
������CxHy��������ȣ������������Խ����ȫȼ�պ���Խ�࣮��
Խ������Խ��
A��C2H6��C��Hԭ����Ŀ֮��=1��3��
B��C2H4��C��Hԭ����Ŀ֮��=1��2
C��C4H10��C��Hԭ����Ŀ֮��=1��2.5
D��C5H10��C��Hԭ����Ŀ֮��=1��2
��������H�������������������ȣ��������������������飬
�ʴ�Ϊ��A��
������Ӧǰ��150�棩��������ʵ����������仯������CxHy��Hԭ����ĿΪ4��ѡ����ֻ��C2H4���ϣ��ʴ�Ϊ��B��
��2���������������䣬��O2�����䣬���ʽ��ͬ�������㣬C2H4��C3H6���ʽ����CH2��HCHO��CH3COOH�����ʽ����HCHO���������⣬
����ˮ���������䣬�������HԪ������������ȣ����ʽ��ͬHԪ������������ȣ�E��C8H10��C4H7��OH��3�е�H������������ͬ��C�����ʼ�D������HԪ��������������ȣ�����ABE���ϣ�
�ʴ�Ϊ��AB��ABE��
�����ܵ����ʵ������䣬���O2�����䣬��������x+
-
����ȣ�
A��1molC2H4��1molC3H6�������x+
�������
B��1molHCHO��1molCH3COOH�������x+
-
������ȣ�
C��CH3COOH��дΪCH4��CO2����CH4�ĺ�������ȣ�
D��CH3CH2OH��дΪC2H4��H2O����CH2�TCH2�ĺ�������ȣ�
E��C8H10��C4H7��OH��3����������ȣ�
���ܵ����ʵ������䣬ֻ��CD��������ȣ�
����ˮ���������䣬�������HԪ������ͬ��C��E����ԭ������ͬ��������ˮ���������䣻
����CO2���������䣬�������Cԭ����Ŀ��ȣ�ѡ����ֻ��D���ϣ�
�ʴ�Ϊ��CD��C��E��D��
| y |
| 4 |
| y |
| 4 |
�ʴ�Ϊ��D��
������CxHy��������ȣ������������Խ����ȫȼ�պ���Խ�࣮��
| y |
| x |
A��C2H6��C��Hԭ����Ŀ֮��=1��3��
B��C2H4��C��Hԭ����Ŀ֮��=1��2
C��C4H10��C��Hԭ����Ŀ֮��=1��2.5
D��C5H10��C��Hԭ����Ŀ֮��=1��2
��������H�������������������ȣ��������������������飬
�ʴ�Ϊ��A��
������Ӧǰ��150�棩��������ʵ����������仯������CxHy��Hԭ����ĿΪ4��ѡ����ֻ��C2H4���ϣ��ʴ�Ϊ��B��
��2���������������䣬��O2�����䣬���ʽ��ͬ�������㣬C2H4��C3H6���ʽ����CH2��HCHO��CH3COOH�����ʽ����HCHO���������⣬
����ˮ���������䣬�������HԪ������������ȣ����ʽ��ͬHԪ������������ȣ�E��C8H10��C4H7��OH��3�е�H������������ͬ��C�����ʼ�D������HԪ��������������ȣ�����ABE���ϣ�
�ʴ�Ϊ��AB��ABE��
�����ܵ����ʵ������䣬���O2�����䣬��������x+
| y |
| 4 |
| z |
| 2 |
A��1molC2H4��1molC3H6�������x+
| y |
| 4 |
B��1molHCHO��1molCH3COOH�������x+
| y |
| 4 |
| z |
| 4 |
C��CH3COOH��дΪCH4��CO2����CH4�ĺ�������ȣ�
D��CH3CH2OH��дΪC2H4��H2O����CH2�TCH2�ĺ�������ȣ�
E��C8H10��C4H7��OH��3����������ȣ�
���ܵ����ʵ������䣬ֻ��CD��������ȣ�
����ˮ���������䣬�������HԪ������ͬ��C��E����ԭ������ͬ��������ˮ���������䣻
����CO2���������䣬�������Cԭ����Ŀ��ȣ�ѡ����ֻ��D���ϣ�
�ʴ�Ϊ��CD��C��E��D��
���������⿼���л���ȼ���йؼ��㣬��Ŀ�Ѷ��еȣ�ע��Թ��ɵ��������գ������ڿ���ѧ���ķ��������ͼ���������

��ϰ��ϵ�д�
�����Ŀ
���г����ʣ�������Ϊ���ʣ������������ǣ�������
| A��Fe3+��Fe2+�����ӹ������ۣ����� |
| B��Mg2+��Al3+�����ӹ�����ˮ������ |
| C��CO2��HCl����ͨ��̼���Ʊ�����Һ��ϴ�� |
| D������Na2CO3��NaHCO3�������������� |
�����������ʵ����ͬһԭ�����͵��ǣ�������
| A���Ȼ��ƺ��Ȼ�þ��Һ�ֱ�����������Һ��϶��ܲ�����ɫ���� |
| B��Ũ�����ϡ���᳤�ڱ�¶�ڿ�����Ũ�Ƚ��� |
| C����ˮ�ͻ���̿ʹ��īˮ��ɫ |
| D��Ư�ۺ�ˮ�������ڱ�¶�ڿ����б��� |
ij�����廯����A�ķ���ʽΪC8H10O�����Ľṹ������������-CH3����������FeCl3��Һ�ɷ�����ɫ��Ӧ�������Ľṹ���У�������
| A��4�� | B��5�� | C��6�� | D��7�� |
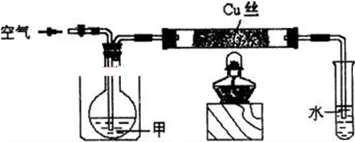
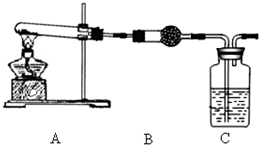
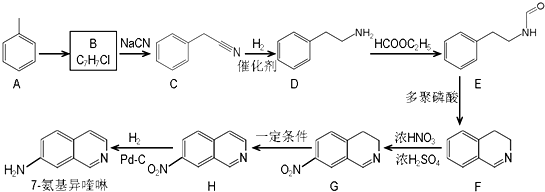
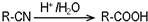 ����ش��������⣺
����ش��������⣺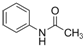 ��������ƺϳ�·�ߣ����Լ����ܼ���ѡ����
��������ƺϳ�·�ߣ����Լ����ܼ���ѡ����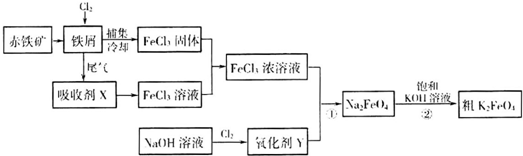
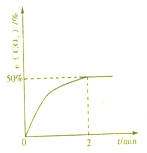 ������CO��SO2�̵�����Ⱦ��һ�ַ������ǽ����ڴ���������ת��Ϊ����S��g����������ӦΪ��2CO��g��+SO2��g��?S��g��+2CO2��g��
������CO��SO2�̵�����Ⱦ��һ�ַ������ǽ����ڴ���������ת��Ϊ����S��g����������ӦΪ��2CO��g��+SO2��g��?S��g��+2CO2��g��