��Ŀ����
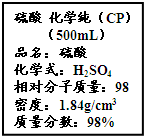 ��Ҫ���������С�⣮
��Ҫ���������С�⣮��1��0.5mol H2O������Ϊ
��2��0.01molij���ʵ�����Ϊ1.08g��������ʵ�Ħ������Ϊ
��3������50mL 0.2mol/L CuSO4��Һ����ҪCuSO4g����ҪCuSO4?5H2O
��4����ͼ����У��ѧʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ�
�ٸ�Ũ��������ʵ���Ũ��
���ø�Ũ��������200mL1mol/L��ϡ���ᣬ��Ͳ������ȡ��Ũ����������
���㣺���ʵ���Ũ�ȵ���ؼ���,�����ӵ�����
ר�⣺���ʵ���Ũ�Ⱥ��ܽ��ר��
��������1������m=nM����0.5mol ˮ������������N=nNA����ˮ������Ŀ��ˮ���Ӻ���10�����ӣ�������Ŀ��ˮ������Ŀ��10����
��2������m=
���㣻
��3������n=cV��������ͭ���ʵ���������ͭ���ʵ�����������ͭ��������ʵ���������m=nM�������������
��4���ٸ���c=
���㣻
�ڸ���ϡ�Ͷ��ɼ���Ũ����������
��2������m=
| m |
| n |
��3������n=cV��������ͭ���ʵ���������ͭ���ʵ�����������ͭ��������ʵ���������m=nM�������������
��4���ٸ���c=
| 1000w |
| M |
�ڸ���ϡ�Ͷ��ɼ���Ũ����������
���
�⣺��1��0.5mol H2O������=0.5mol��18g/mol=9g�����з�����Ŀ=0.5mol��NAmol-1=0.5NA��ˮ���Ӻ���10�����ӣ�������Ŀ��ˮ������Ŀ��10���������е�����ĿΪ5NA��
�ʴ�Ϊ��9��0.5NA��5NA��
��2��0.01molij���ʵ�����Ϊ1.08g��������ʵ�Ħ������Ϊ
=108g/mol��
�ʴ�Ϊ��108g/mol��
��3������50mL 0.2mol/L CuSO4��Һ��Ҫ����ͭ�����ʵ���=0.05L��0.2mol/L=0.01mol������Ҫ����ͭ������=0.01mol��160g/mol=1.6g������ͭ���ʵ�����������ͭ��������ʵ�������ҪCuSO4?5H2O ������=0.01mol��250g/mol=2.5g��
�ʴ�Ϊ��1.6��2.5��
��4������������98%���ܶ�1.84g/mL��Ũ��������ʵ���Ũ��=
mol/L=18.4mol/L��
�ʴ�Ϊ��18.4mol/L��
���ø�Ũ��������200mL1mol/L��ϡ���ᣬ����ҪŨ��������ΪV mL������ϡ�Ͷ��ɣ���
0.2L��1mol/L=V��10-3 L��18.4 mol/L
���V=10.9
�ʴ�Ϊ��10.9��
�ʴ�Ϊ��9��0.5NA��5NA��
��2��0.01molij���ʵ�����Ϊ1.08g��������ʵ�Ħ������Ϊ
| 1.08g |
| 0.01mol |
�ʴ�Ϊ��108g/mol��
��3������50mL 0.2mol/L CuSO4��Һ��Ҫ����ͭ�����ʵ���=0.05L��0.2mol/L=0.01mol������Ҫ����ͭ������=0.01mol��160g/mol=1.6g������ͭ���ʵ�����������ͭ��������ʵ�������ҪCuSO4?5H2O ������=0.01mol��250g/mol=2.5g��
�ʴ�Ϊ��1.6��2.5��
��4������������98%���ܶ�1.84g/mL��Ũ��������ʵ���Ũ��=
| 1000��1.84��98% |
| 98 |
�ʴ�Ϊ��18.4mol/L��
���ø�Ũ��������200mL1mol/L��ϡ���ᣬ����ҪŨ��������ΪV mL������ϡ�Ͷ��ɣ���
0.2L��1mol/L=V��10-3 L��18.4 mol/L
���V=10.9
�ʴ�Ϊ��10.9��
���������⿼�����ʵ������йؼ��㣬�ѶȲ���ע��Թ�ʽ�����������Ӧ�ã�ע���������ʵ���Ũ�������������Ĺ�ϵ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
���е���в����ڶ��ε�ص��ǣ�������
| A������п�̵�� | B��Ǧ���� |
| C�����ӵ�� | D������ӵ�� |
X��Y��Z��W�Ƕ�����Ԫ�أ�XΪ�ؿ��к�����ߵĽ���Ԫ�أ�Yԭ�������������ǵ���������
��Z����������ϼ���������ϼ۵Ĵ�����Ϊ4��Wԭ���ڶ�������ԭ�Ӱ뾶�������˵����ȷ���ǣ�������
| 2 |
| 3 |
| A��ZO3��ˮ��Ӧ�γɵĻ����������ӻ����� |
| B��ճ���Թ��ڱ��ϵ�Z������YZ2ϴ�� |
| C������������Ӧˮ����ļ��ԣ�X��W |
| D��Xλ�ڽ�����ǽ����ķֽ��ߴ������������뵼����� |
 ��1������ʽΪC4H10O�Ĵ�����
��1������ʽΪC4H10O�Ĵ�����
 A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C����Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������B2-�Ľṹʾ��ͼΪ
A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C����Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������B2-�Ľṹʾ��ͼΪ �����û�ѧ����ش��������⣺
�����û�ѧ����ش��������⣺