��Ŀ����
5�� ����������⣬�������С�⣮
����������⣬�������С�⣮��1����֪H2O2���ӵĽṹ��ͼ��ʾ��H2O2���Ӳ���ֱ���εģ�������ԭ�������ڰ�չ������������ϣ�������ԭ�����鼹λ���ϣ���ҳ�н�Ϊ93��52�䣬������O-H����O-O���ļнǾ�Ϊ96��52�䣮
�Իش�
��H2O2�ṹʽ��H-O-O-H��
��H2O2�����Ǻ��м��ԣ�����ԡ��Ǽ��ԡ������ӣ�
��H2O2�����ڷǼ����ܼ�CS2����Ҫ˵��H2O2Ϊ���Է��ӣ���CS2Ϊ�Ǽ����ܼ������ݡ��������ܡ����ɣ�H2O2������CS2��
��2��3-��-2-������Ľṹ��ʽΪ��
 ��һ�����л�������к���2������̼ԭ�ӣ�����һ�Զ�ӳ�칹���ü�ͶӰʽ��ʾΪ��
��һ�����л�������к���2������̼ԭ�ӣ�����һ�Զ�ӳ�칹���ü�ͶӰʽ��ʾΪ�� ��
�� ������һ�Զ�ӳ�칹��ļ�ͶӰʽΪ��
������һ�Զ�ӳ�칹��ļ�ͶӰʽΪ�� ��
�� ���ڢ�
���ڢ�
���� ��1���ٸ��ݽṹʽ�Ķ��������һ�Թ��õ��Ӷ���һ����������ʾ��
��ͬ��Ԫ��ԭ�Ӽ��γɷǼ��Լ�����ͬ��Ԫ��ԭ�Ӽ��γɼ��Լ���H2O2���Ӳ���ֱ���εģ�������ԭ�������ڰ�չ������������ϣ�����������IJ��غϣ����ӱ���Ϊ���Է��ӣ�
���ж�H2O2��CS2���ӵļ��ԣ������������ܵ�ԭ�����
��2������4����ͬԭ�ӻ�ԭ���ŵ�̼ԭ��Ϊ����̼ԭ�ӣ��ݴ��ص��жϼ��ɣ���������֪������̼ԭ������4����ͬ��ԭ�ӻ�ԭ���ţ���������̼ԭ�ӵ������������������ԣ����ڶ�ӳ�칹�壬�������칹��
��� �⣺��1����һ�Թ��õ��Ӷ���һ����������ʾ�����ԽṹʽΪ��H-O-O-H���ʴ�Ϊ��H-O-O-H��
��˫��ˮ���������γɵ��Ǽ��Լ����������γɵ��ǷǼ��Լ���H2O2���Ӳ���ֱ���εģ�������ԭ�������ڰ�չ������������ϣ�������ԭ�����鼹λ���ϣ���ҳ�н�Ϊ93��52�䣬������O-H����O-O���ļнǾ�Ϊ96��52�䣬������������ɲ��غ��γɵ��Ǽ��Է��ӣ��ʴ�Ϊ�����ԣ�
����������ԭ����ָ���ڼ��Է��Ӽ�ĵ������ã�ʹ�ü��Է�����ɵ����������ڼ��Է�����ɵ��ܼ��������ڷǼ��Է�����ɵ��ܼ����Ǽ��Է�����ɵ����������ڷǼ��Է�����ɵ��ܼ��������ڼ��Է�����ɵ��ܼ���H2O2�Ǽ��Է��ӣ�CS2�ǷǼ��Է��ӣ������������ܵ�ԭ����֪��H2O2������CS2��
�ʴ�Ϊ����H2O2Ϊ���Է��ӣ���CS2Ϊ�Ǽ����ܼ���������������ԭ����H2O2������CS2�У�
��2������4����ͬԭ�ӻ�ԭ���ŵ�̼ԭ��Ϊ����̼ԭ�ӣ�3-��-2-������Ľṹ��ʽΪ�� ��һ�����л�������к���2������̼ԭ�ӣ�
��һ�����л�������к���2������̼ԭ�ӣ�
��ӳ�칹���ü�ͶӰʽ��ʾΪ�� ��
�� ������һ�Զ�ӳ�칹��ļ�ͶӰʽΪ��
������һ�Զ�ӳ�칹��ļ�ͶӰʽΪ�� ��
�� ��
��
�ʴ�Ϊ��2�� ��
��
���� ���⿼����˫��ˮ�Ľṹ�����ʣ�����̼ԭ�ӵ��жϺͶ�Ӧ�칹�����д��ע��˫��ˮ�м��ļ��Ժͷ��ӵļ��ԣ�����̼ԭ���ж�ע�⣺����̼ԭ��һ���DZ���̼ԭ�ӣ���Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | 1mol�����к���6NA�����ۼ� | |
| B�� | 1mol�Ҵ�������������Ũ������·�Ӧ������1mol�������� | |
| C�� | 1molij���ʹ����ɿ����������е��ⱻ�ǻ�ȡ���IJ���������Ľ����Ʒ�Ӧ����1molH2��˵����һ�������к��������ǻ� | |
| D�� | 1mol��ϩ�����ӳɷ�Ӧ�����2mol���ۼ� |
| A�� | mol | B�� | g/mol | C�� | mol/L | D�� | mol/L/s |
| A�� | 5��1 | B�� | 1��5 | C�� | 6��1 | D�� | 1��6 |
| A�� | �����ܲ��������ЧӦ | B�� | ���岻��ͨ����ֽ | ||
| C�� | ������۲����� | D�� | ���岻�ȶ������ú����ײ������� |
| A�� | HCO3-+H2O?H3O++CO32- | B�� | PO43-+3H2O?H3PO4+3OH- | ||
| C�� | NH4++H2O?NH3H2O+H+ | D�� | H2O+H2O?H3O++OH- |
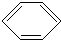 +Cl2
+Cl2
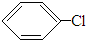 +HCl
+HCl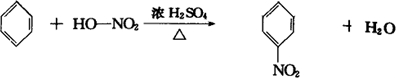

 ��
�� +2CH3OH$\stackrel{һ������}{��}$CH3OCOOCH3+HOCH2CH2OH��
+2CH3OH$\stackrel{һ������}{��}$CH3OCOOCH3+HOCH2CH2OH�� ��д�ṹ��ʽ��
��д�ṹ��ʽ�� ��
��