��Ŀ����
5�� ����ˮ����ζ����������A���Է������б仯��
����ˮ����ζ����������A���Է������б仯����1�����е�������CH3COOH��G�Ľṹ��ʽΪCH2=CH2
��2��д�����б仯�ķ���ʽ��
A+NaOH��B+C�Ļ�ѧ����ʽ��CH3COOCH2CH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3CH2OH
C��D�Ļ�ѧ����ʽ��2C2H5OH+O2$��_{��}^{ͭ����}$2CH3CHO+2H2O
D��������Ӧ�����ӷ���ʽ��CH3CHO+2Ag��NH3��2++2OH-$\stackrel{ˮԡ}{��}$CH3COO-+NH4++2Ag��+3NH3+H2O��
���� ����ˮ����ζ����������A�ܷ���ˮ�ⷴӦ����B��C��B�����ᷴӦ����һ�����ᣬ��B�������ƣ�C�Ǵ���C����������D��D�ܷ���������Ӧ����DΪȩ��D��������������E��E���������Ʒ�Ӧ����������B��������ʹ�������Cԭ����ȣ�C������ȥ��Ӧ����G��G���巢���ӳɷ�Ӧ����1��2-�������飬��G�ṹ��ʽΪCH2=CH2��C�ṹ��ʽΪCH3CH2OH��DΪCH3CHO��EΪCH3COOH��BΪCH3COONa��AΪCH3COOCH2CH3���ݴ˷������
��� �⣺����ˮ����ζ����������A�ܷ���ˮ�ⷴӦ����B��C��B�����ᷴӦ����һ�����ᣬ��B�������ƣ�C�Ǵ���C����������D��D�ܷ���������Ӧ����DΪȩ��D��������������E��E���������Ʒ�Ӧ����������B��������ʹ�������Cԭ����ȣ�C������ȥ��Ӧ����G��G���巢���ӳɷ�Ӧ����1��2-�������飬��G�ṹ��ʽΪCH2=CH2��C�ṹ��ʽΪCH3CH2OH��DΪCH3CHO��EΪCH3COOH��BΪCH3COONa��AΪCH3COOCH2CH3��
��1��ͨ�����Ϸ���֪�����е�������CH3COOH��G�Ľṹ��ʽΪCH2=CH2��
�ʴ�Ϊ��CH3COOH��CH2=CH2��
��2��A������������A���������Ƶķ�Ӧ����ʽΪCH3COOCH2CH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3CH2OH��C��D�Ļ�ѧ����ʽΪ2C2H5OH+O2$��_{��}^{ͭ����}$2CH3CHO+2H2O��DΪCH3CHO��D��������Ӧ�����ӷ���ʽΪCH3CHO+2Ag��NH3��2++2OH- $\stackrel{ˮԡ}{��}$ CH3COO-+NH4++2Ag��+3NH3+H2O��
�ʴ�Ϊ��CH3COOCH2CH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3CH2OH��2C2H5OH+O2$��_{��}^{ͭ����}$2CH3CHO+2H2O��CH3CHO+2Ag��NH3��2++2OH- $\stackrel{ˮԡ}{��}$ CH3COO-+NH4++2Ag��+3NH3+H2O��
���� ���⿼���л����ƶϣ����ؿ�������ƶ��������漰����ȩ�����ᡢ��֮���ת�������ݷ�Ӧ����ȷ�����ʾ��еĹ����ż���ṹ���������ճ����л���Ӧ���ͣ���Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 2 min | B�� | 3 min | C�� | 4 min | D�� | 5 min |
| A�� | ��Ӧ�У�N2�ǻ�ԭ����Al2O3�������� | |
| B�� | ��Ӧ�У�ÿ����2 mol AlN��N2�õ�3 mol���� | |
| C�� | �������е�Ԫ�صĻ��ϼ�Ϊ-3 | |
| D�� | AlN��Ħ������Ϊ41g |
| ѡ�� | A | B | C | D |
| Ŀ�� | ʵ������O2 | ����ϡ������Һ | ������Ʒ�϶�ͭ | ���װ�������� |
| װ�û���� | 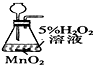 | 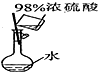 | 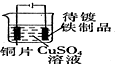 | 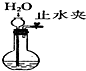 |
| A�� | A | B�� | B | C�� | C | D�� | D |
��ʯ�ͷ���ʱ���¶ȼƲ������ȵ�Һ����
���ñ���̼������Һ��ȥ���������л��е��Ҵ������ᣮ
�������Ը��������Һ��ȥ��Ȳ�е���ϩ
�ܽ�������ˮ��Ϻ����������ȡ�屽��
| A�� | ֻ�Т� | B�� | ֻ�Т٢ۢ� | C�� | ֻ�Т٢ڢ� | D�� | �٢ڢۢ� |
| A�� | Fe��OH��3 | B�� | Fe��OH��2 | C�� | FeCl2 | D�� | FeCl3 |
| NaCl | MgCl2 | AlCl3 | SiCl4 | ����B | |
| �۵�/�� | 810 | 710 | 190 | -68 | 2300 |
| �е�/�� | 1465 | 1418 | 182.7 | 57 | 2500 |
| A�� | SiCl4�Ƿ��Ӿ��� | B�� | MgCl2�������Ӽ���ǿ�ȱ�NaCl�� | ||
| C�� | AlCl3���������� | D�� | ����B��ԭ�Ӿ��� |
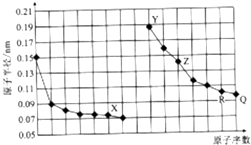
| A�� | QX2��RX2����ɱ�����������ã����߳���������ˮ���� | |
| B�� | �����ӵİ뾶��X��Y��Z | |
| C�� | Y��Z��R��Ӧ������������֮����Է�����Ӧ | |
| D�� | ������ڵ�X��Z���ɵĻ�������Եõ�����Z |