��Ŀ����
Ԫ��Xλ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ2��Ԫ��Y��̬ԭ�ӵ�3p�������4�����ӣ�Ԫ��Z��ԭ�����������������ڲ��3����
��1����Y���⻯�H2Y�������У�Yԭ�ӹ�����ӻ������� ��
��2��Y��Z���γ�YZ42-
��YZ42-�Ŀռ乹��Ϊ ����������������
��д��һ����YZ42-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽ�� ��
��3��X���Ȼ����백ˮ��Ӧ���γ������[X��NH3��4]Cl2��1mol��������к�����λ������ĿΪ ��
��1����Y���⻯�H2Y�������У�Yԭ�ӹ�����ӻ�������
��2��Y��Z���γ�YZ42-
��YZ42-�Ŀռ乹��Ϊ
��д��һ����YZ42-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽ��
��3��X���Ȼ����백ˮ��Ӧ���γ������[X��NH3��4]Cl2��1mol��������к�����λ������ĿΪ
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������Ԫ��X λ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ����ڲ������=2+8+18=28��������������Ϊ2�����Ը�ԭ����30�����ӣ�ΪZnԪ�أ�Ԫ��Y��̬ԭ�ӵ�3p�������4�����ӣ���Y��SԪ�أ�Ԫ��Z��ԭ�����������������ڲ��3����Ԫ������������С�ڻ����8������Z��OԪ�أ��ݴ˽���С�⼴�ɣ�
���
�⣺���ݷ�����֪��XΪп��YΪ��ZΪ����
��1��H2S�����У�Sԭ�ӵļ۲������=
=4������Sԭ�ӵĹ�����ӻ�������sp3�ӻ���
�ʴ�Ϊ��sp3��
��2����SO42-��Sԭ�ӵļ۲������=4+
��6+2-4��2��=4����û�йµ��Ӷԣ���������������ṹ��
�ʴ�Ϊ���������壻
��YZ42-ΪSO42-���京�������Ϊ��16+4��8+2=50������ȵ�����ķ����У�CCl4��SiCl4���ʴ�Ϊ��CCl4��SiCl4��
��3�������[Zn��NH3��4]Cl2�У�п�����백����֮����4����λ����ÿ������������3�����ۼ�������1mol��������к�����λ������ĿΪ4��6.02��1023�����ʴ�Ϊ��4��6.02��1023����
��1��H2S�����У�Sԭ�ӵļ۲������=
| 6+2 |
| 2 |
�ʴ�Ϊ��sp3��
��2����SO42-��Sԭ�ӵļ۲������=4+
| 1 |
| 2 |
�ʴ�Ϊ���������壻
��YZ42-ΪSO42-���京�������Ϊ��16+4��8+2=50������ȵ�����ķ����У�CCl4��SiCl4���ʴ�Ϊ��CCl4��SiCl4��
��3�������[Zn��NH3��4]Cl2�У�п�����백����֮����4����λ����ÿ������������3�����ۼ�������1mol��������к�����λ������ĿΪ4��6.02��1023�����ʴ�Ϊ��4��6.02��1023����
������������Ҫ������ԭ���ӻ���ʽ�����ӵĿռ乹�͡��ȵ������֪ʶ���ѶȲ���Ԫ���ƶ��ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ





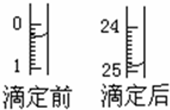 ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ����ⶨ��Ũ�ȣ�
ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ����ⶨ��Ũ�ȣ�