��Ŀ����
14����ͼΪ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�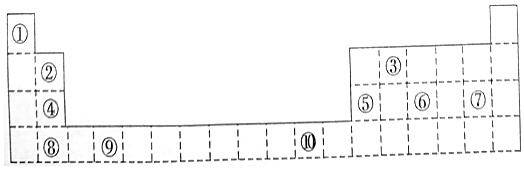
��ش��������⣺
��1����������d��Ԫ�ص���Ti����Ԫ�ط��ţ���
��2���͢��γɵķ����и���ԭ�Ӿ�����8�����ȶ��ṹ�������幹��Ϊ�����Σ�
��3��ijԪ��ԭ�ӵ���Χ�����Ų�ʽΪnsnnpn+1����Ԫ��ԭ�Ӻ͢��γɵķ��ӣ�����ԭ���ϵļ۲���Ӷ���Ϊ4��
��4��Ԫ�آߺ͢��γɵĻ�����ĵ���ʽΪ
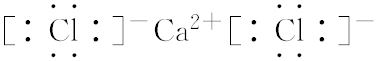 ��
����5��Ԫ�آ�Ļ�̬+2�����ӵĵ����Ų�ʽ��1s22s22p63s23p63d9��[Ar]3d9��
��6��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آݵ��������������Ƶ����ʣ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��Be��OH��2+2NaOH�TNa2BeO2+2H2O��
���� ��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪBe����ΪC����ΪMg����ΪAl����ΪP����ΪCl����ΪCa����ΪTi����ΪCu��
��1��d��Ԫ���ڸ���Ԫ�غ���Ԫ������
��2��P��Cl�γ�PCl3��PCl5�����ݸ�Ԫ�ػ��ϼ۵ľ���ֵ��������������ӵ���8�жϳ�PCl3����Ҫ������۲���Ӷ��������ж����幹�ͣ�
��3�������Ų�ʽΪnsnnpn+1��nֻ��Ϊ2����Χ�����Ų�ʽΪ��2s22p3�����Ԫ��ΪN����Cl�γɵķ���ΪNCl3��
��4���Ȼ��������ӻ�������ݵ���ʽ����д���ش�
��5��Cu�ĺ��������29����д����̬ԭ�Ӻ�������Ų�ʽ����д�����������ӵĵ����Ų�ʽ��
��6��Ԫ�آڵ�����������NaOH��Һ��Ӧ��Ӧ����Na2BeO2��
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪBe����ΪC����ΪMg����ΪAl����ΪP����ΪCl����ΪCa����ΪTi����ΪCu��
��1��d��Ԫ���ڸ���Ԫ�غ���Ԫ������Cuλ��ds����ֻ��Tiλ��d����
�ʴ�Ϊ��Ti��
��2��P��Cl�γ�PCl3��PCl5�������и���ԭ���ܾ�����8�����ȶ��ṹ����PCl3���۲���Ӷ���Ϊ3+$\frac{5-3��1}{2}$=4���¶Ե���1�ԣ������幹��Ϊ�����Σ�
�ʴ�Ϊ�������Σ�
��3�������Ų�ʽΪnsnnpn+1��nֻ��Ϊ2����Χ�����Ų�ʽΪ��2s22p3�����Ԫ��ΪN����Cl�γɵķ���ΪNCl3���۲���Ӷ���Ϊ3+$\frac{5-3��1}{2}$=4��
�ʴ�Ϊ��4��
��4��Cl��Ca�γɵĻ������Ȼ��������ӻ��������ʽΪ��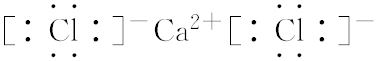 ��
��
�ʴ�Ϊ��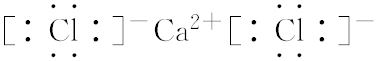 ��
��
��5��Ԫ�آ�ΪCu�����������29�������Ļ�̬ԭ�Ӻ�������Ų�ʽ�ǣ�1s22s22p63s23p63d104s1��[Ar]3d104s1�����������ӵĵ����Ų�ʽΪ��1s22s22p63s23p63d9��[Ar]3d9��
�ʴ�Ϊ��1s22s22p63s23p63d9��[Ar]3d9��
��6��Ԫ�آڵ�����������NaOH��Һ��Ӧ��Ӧ����Na2BeO2���÷�ӦΪBe��OH��2+2NaOH�TNa2BeO2+2H2O��
�ʴ�Ϊ��Be��OH��2+2NaOH�TNa2BeO2+2H2O��
���� ���⿼��λ�á��ṹ�����ʵ�Ӧ�ã�Ϊ��Ƶ���㣬�漰Ԫ�����ڱ���Ԫ�صķ�����Ԫ�����ڱ���Ԫ�ص�λ�á����ӹ��͡���ѧ��Ӧ�ȣ��ۺ��Խ�ǿ����Ŀ�ѶȽϴ�

 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д� ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�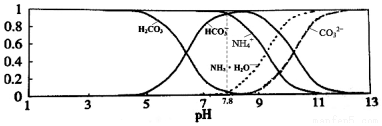
| A�� | ����Һ��pH=9ʱ����Һ�д������й�ϵ��c��NH4+����c��HCO3-����c��NH3•H2O����c��CO32-�� | |
| B�� | ������Һ����εμ���������ʱNH4+��HCO3-Ũ����С | |
| C�� | NH4HCO3��Һ�У�c��NH4+��+c��NH3•H2O��+c��H+��=c��CO32-��+c��H2CO3��+c��HCO3-��+c��OH-�� | |
| D�� | ͨ��������֪������Kb��NH3•H2O����Ka1��H2CO3�� |
| A�� | SCN- | B�� | CO32- | C�� | Cl- | D�� | OH- |
| A�� | NH4+��SO32-��AlO2- | B�� | NH4+��Br-��CO32- | ||
| C�� | Fe2+��S2-��SO32- | D�� | NH4+��Br-��AlO2- |
| A | B | C | D | E |
| A�� | HnDOmΪǿ��ʱ��E�ķǽ�����һ����ǿ | |
| B�� | A��OH��nΪǿ��ʱ��B��OH��mҲһ��Ϊǿ�� | |
| C�� | EԪ�ص�����ϼ�Ϊ+7ʱ��DԪ�صĸ����ϼۿ�Ϊ-2�� | |
| D�� | HnCOmΪǿ��ʱ��E�ĵ��ʿ�����ǿ��ԭ�� |
| A�� | ������̿��CO2�ķ�Ӧ | B�� | Ba��OH��2•8H2O��NH4Cl�ķ�Ӧ | ||
| C�� | ��ʯ������ˮ�ķ�Ӧ | D�� | �������ռ���Һ���кͷ�Ӧ |
| A�� | ���顢���顢�����ڹ����·ֱ���������Ӧ�����ɵ�һ�ȴ��ﶼֻ��һ�� | |
| B�� | ���ü�ѹ���˵ķ��������������ٷ��롢�ᴿ������ | |
| C�� | ������ˮ��ϣ���������ˮ����ɫ���������ڷ�����ȡ����Ӧ | |
| D�� | �ڶ��ױ�ֻ��һ�ֽṹ��֤�������в�����̼̼������̼̼˫������Ľṹ |
 ��֪NO2����SO2�ܷ�����Ӧ��NO2��g��+SO2��g��?SO3��g��+NO��g����
��֪NO2����SO2�ܷ�����Ӧ��NO2��g��+SO2��g��?SO3��g��+NO��g���� ��
�� �������
����д����  ת��Ϊ
ת��Ϊ  �Ļ�ѧ����ʽ
�Ļ�ѧ����ʽ +H2O+CO2����
+H2O+CO2����
 ��������������������Һ������Ӧ�ķ���ʽ��
��������������������Һ������Ӧ�ķ���ʽ�� ��
��