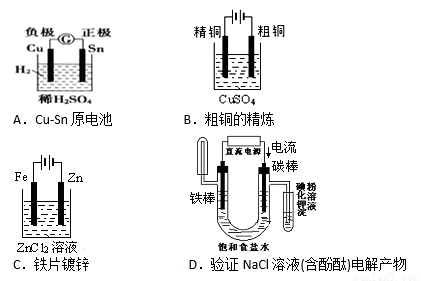
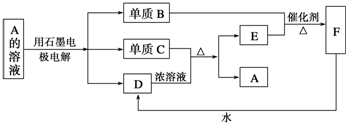
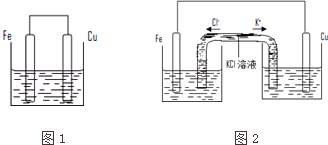
��Ŀ����
12��������Ǹ�ϰ�˳����ļ��ֽ�����Na��Mg��Al��Fe��Cu������������ѧ֪ʶ�ش��������⣺��1����ԭ�Ӻ��������11�˶�״̬���仯����Na2O2��������������
��2��þ�ڶ�����̼�е�ȼ�Ļ�ѧ����ʽΪ2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
��3������Ԫ�����ڱ���λ�ڵ������ڵ�IIIA�壮
��4����ȥFe�е�����Al��������Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
��5�����漸�ֽ����п����Ȼ�ԭ��ұ������Fe��Cu��
��6��ͭ�ļ۵����Ų�ʽΪ3d104s1������3d94s2��������Ϊ[Ar]3d104s1��d�������ȫ����s������ڰ�����
���� ��1��ԭ�Ӻ����м������ӣ����м����˶�״̬��Na2O2����ˮ��������̼��Ӧ����������
��2��þ�ڶ�����̼�е�ȼ����MgO��C��
��3����ԭ�Ӻ�����3�����Ӳ㣬�������3�����ӣ�
��4����ȥFe�е�����Al������������Һ��
��5��Na��Mg��Al�õ�ⷨұ����Fe��Cu���Ȼ�ԭ��ұ����
��6��ԭ�ӹ���ϵ����Ų�Ϊ��ȫ�ա���������������ȫ������ʱ��һ������ȶ���
��� �⣺��1����ԭ�Ӻ��������11�����ӣ�ԭ�Ӻ����м������ӣ����м����˶�״̬��������ԭ�Ӻ��������11�˶�״̬��Na2O2����ˮ��������̼��Ӧ������������������������
�ʴ�Ϊ��11����������
��2��þ�ڶ�����̼�е�ȼ����MgO��C���䷴Ӧ�Ļ�ѧ����ʽΪ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
�ʴ�Ϊ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
��3����ԭ�Ӻ�����3�����Ӳ㣬�������3�����ӣ���AlԪ�������ڱ���λ�ڵ������ڵ�IIIA�壻
�ʴ�Ϊ���������ڵ�IIIA�壻
��4����ȥFe�е�����Al������������Һ��Al��������������Һ��Fe���ܣ��䷴Ӧ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��5��Na��Mg��Al�õ�ⷨұ����Fe��Cu���Ȼ�ԭ��ұ����
�ʴ�Ϊ��Fe��Cu��
��6��ԭ�ӹ���ϵ����Ų�Ϊ��ȫ�ա���������������ȫ������ʱ��һ������ȶ�����̬ͭ��Cu��ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s1������[Ar]3d94s2������Ϊ[Ar]3d104s1��d�������ȫ����s������ڰ�����
�ʴ�Ϊ����Ϊ[Ar]3d104s1��d�������ȫ����s������ڰ�����
���� ���⿼���˵����Ų�����ѧ����ʽ��Ԫ�����ڱ���������ұ���ȣ���Ŀ�Ѷ��еȣ������ڿ���ѧ���Ի���֪ʶ��Ӧ��������ע����յ����Ų����ɣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�������ȼ���ױ����ж��Ļ�ѧ���ʣ������������װ����Σ�վ����ǩ���������������У������˱�ǩ����
| A | B | C | D |
���ʵĻ�ѧʽ | H2SO4(Ũ) | C2H5OH(�ƾ�) | Hg(��) | NaCl |
Σ�վ����־ |
|
|
|
|
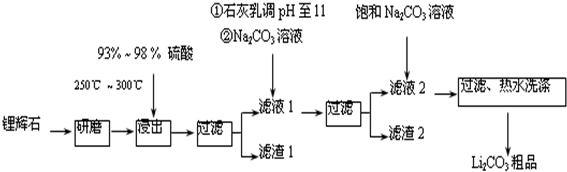
��֪����Li2O•Al2O3•4SiO2+H2SO4��Ũ��$\frac{\underline{\;250��-300��\;}}{\;}$Li2SO4+Al2O3•4SiO2•H2O��
��ijЩ���ʵ��ܽ�ȣ�S�������ʾ��
| T/�� | 20 | 40 | 60 | 80 |
| S��Li2CO3��/g | 1.33 | 1.17 | 1.01 | 0.85 |
| S��Li2SO4��/g | 34.2 | 32.8 | 31.9 | 30.7 |
��2����֪����2����Ҫ�ɷ���Mg��OH��2��CaCO3��
����Һ1�м���ʯ����������ǣ����û�ѧƽ���ƶ�ԭ�����ͣ�����Ca2+��OH-��Ũ�ȣ�������Mg��OH��2��CaCO3��������
��3������Һ2�м��뱥��Na2CO3��Һ�����˺��á���ˮϴ�ӡ���ԭ����Li2CO3���ܽ�����¶����߶���С����ˮϴ�ӿɼ���Li2CO3����ʧ��
��4����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ������£�
a����Li2CO3�������������۵�����Һ��LiOH��Һ������Һ������������ѡ����Ĥ�������ö��Ե缫��⣮
b��������LiOH��Һ�м������NH4HCO3��Һ�����ˡ���ɵøߴ�Li2CO3��
��a�У������ĵ缫��Ӧʽ��2C1--2e-=Cl2����
��b�У�����Li2CO3��Ӧ�Ļ�ѧ����ʽ��2LiOH+NH4HCO3=Li2CO3+NH3+2H2O��
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | CH3CHOHCH3 | B�� | CH2OHCH2CH3 | C�� | ��CH3��2COHCH3 | D�� | ��CH3��3COH |