��Ŀ����
4����������ȵ���Ƭ��ͭƬ�õ�����������500mL����ͭ��Һ�й�����ͼ1��װ�ã�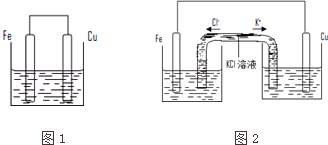
��1��ͭƬ�ϵĵ缫��ӦʽΪCu2++2e-=Cu��ͭƬ��Χ��Һ�������ɫ��dz������
��2������������װ�÷�Ӧһ��ʱ�������Ƭ������2.4g��ͬʱͭƬ������3.2g�������ʱ���ڸ�װ�����ĵĻ�ѧ����50%ת��Ϊ���ܣ�
��3��������װ�ø�Ϊ��ͼ2��ʾ��װ��Ҳ�ܴﵽ��ԭװ����ͬ�����ã�ͬʱ�ܱ�ֹ֤ͣʹ�ø�װ��ʱ��Ӧ�ﲻ��ģ�KCl��Һ��ͨ������Һ�γɱպϻ�·�����ã�������ͭ��ҺӦ��ע���ң����ࡱ�����Ҳࡱ�����ࡱ���ձ��У���2min����Ƭ������5.6g�����м�U�ι���K+��������0.1mo1/min��
��4�������գ�3����װ�÷�Ӧһ��ʱ�������Ƭ��ͭƬ֮���������Ϊ0.6g�������������ĵ���Ϊ0.01mol��
���� ��1��װ���Ƿ�����ӵ�Դ�����ж���������𣬸��ݵ�صĹ���ԭ���������缫�缫��Ӧ�Լ�����
��2�����缫�ĵ���һ���ִ���ͭ�缫������ԭ��ط�Ӧ��ͬʱ���缫��Ҳ�����û���Ӧ���缫��Ӧ��Fe-2e-��Fe2+��Cu2++2e-=Cu��ͭ�缫����3.2g����0.05mol��ͬʱ���缫ԭ��ط�Ӧ����0.05molFe2+�������缫����56g/mol��0.05
mol=2.8g��������Ƭֻ������2.4g����Ϊ��Ƭ��һ����ֱ�ӷ����û���Ӧ����ͭ���ɣ��������������ˣ��ⲿ�����Ļ�ѧ��û��ת��Ϊ���ܣ�������2.8g-2.4g=0.4g�����������xmol��ֱ�Ӳ����û���Ӧ��
Cu2++Fe=Cu+Fe2+��m
1mol 64 8
x 0.4
�����x=0.05mol����ֱ�ӷ����û���Ӧ����Ϊ0.05mol������ԭ��ط�Ӧ����Ҳ��0.05mol��
��3������ԭ����У������缫���뺬�иõ缫���������ӵ�����Һ�У���������ͭ��ҺӦ��ע���Ҳ��ձ��У���2min����Ƭ������5.6g���ö�ʱ�������������ӱ�ʾ��ƽ����Ӧ����v=$\frac{\frac{\frac{5.6g}{56g/mol}}{0.5L}}{2min}$=0.1mol•L-1•min-1�����м�U�ι���K+��������0.1mol/min��
��4�����ݵ缫��Ӧʽ�����㣮
��� �⣺��1��װ��û����ӵ�Դ������ԭ��أ����ý���п������������ʧ���ӣ�Fe-2e-=Fe2+����������ͭ���ӵõ��ӣ��缫��Ӧʽ��Cu2++2e-=Cu������ͭ�����ڸü������ܶȼ�С����Һ��ɫ��dz���ʴ�Ϊ��Cu2++2e-=Cu����ɫ��dz��
��2�����缫�ĵ���һ���ִ���ͭ�缫������ԭ��ط�Ӧ��ͬʱ���缫��Ҳ�����û���Ӧ���缫��Ӧ��Fe-2e-��Fe2+��Cu2++2e-=Cu��ͭ�缫����3.2g����0.05mol��ͬʱ���缫ԭ��ط�Ӧ����0.05molFe2+�������缫����56g/mol��0.05
mol=2.8g��������Ƭֻ������2.4g����Ϊ��Ƭ��һ����ֱ�ӷ����û���Ӧ����ͭ���ɣ��������������ˣ��ⲿ�����Ļ�ѧ��û��ת��Ϊ���ܣ�������2.8g-2.4g=0.4g�����������xmol��ֱ�Ӳ����û���Ӧ��
Cu2++Fe=Cu+Fe2+��m
1mol 64 8
x 0.4
�����x=0.05mol����ֱ�ӷ����û���Ӧ����Ϊ0.05mol������ԭ��ط�Ӧ����Ҳ��0.05mol�����Ը�װ�����Ļ�ѧ����50%ת��Ϊ���ܣ��ʴ�Ϊ��50��
��3������ԭ����У������缫���뺬�иõ缫���������ӵ�����Һ�У���������ͭ��ҺӦ��ע���Ҳ��ձ��У���2min����Ƭ������5.6g���ö�ʱ�������������ӱ�ʾ��ƽ����Ӧ����v=$\frac{\frac{\frac{5.6g}{56g/mol}}{0.5L}}{2min}$=0.1mol•L-1•min-1�����м�U�ι���K+��������0.1mol/min���ʴ�Ϊ���ң�0.1��
��4�����缫�����ķ�ӦΪ��Fe-2e-��Fe2+����������ͭ���ӵõ��ӣ��缫��ӦΪ��Cu2++2e-=Cu����ת�Ƶ���Ϊn��������������28n��Cu�缫��������32n����Ƭ��ͭƬ֮���������Ϊ28n+32n=60n=0.6g������n=0.01���ʴ�Ϊ��0.01��
���� ���⿼��ѧ��ԭ��صĹ���ԭ����ѧ��Ҫѧ��Ӧ�ò������ڵ缫��Ӧ�����е����ã��ۺ���ǿ���ѶȽϴ�

| A�� | ��������ˮ��Cl2+H2O�T2H++Cl-+ClO - | |
| B�� | NaAlO2��Һ��ͨ�������CO2��2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32- | |
| C�� | ��ϡHNO3�ܽ�Fe3O4��������ӷ���ʽ��Fe3O4+8H+�T2Fe3++Fe2++4H2O | |
| D�� | NaHCO3��Һ�м�����Ba��OH��2��Һ��2HCO3-+Ba2++2OH-�TBaCO3��+2H2O+CO32- |
| A�� | Ũ������������������ĥ��������ɫ����ƿ�� | |
| B�� | ���ͻ�ú�ʹ���ڴ���Ƥ������ɫ����ƿ�� | |
| C�� | �ü�ʽ�ζ���ȷ��ȡ25.00mL��KMnO4��Һ | |
| D�� | ������ˮ����������Һ���������ĥ��������ɫ����ƿ�� |
 �������ͣ�
�������ͣ� ���ֱ����������������ã����������������������ִ������ʵ���֮��Ϊ��������
���ֱ����������������ã����������������������ִ������ʵ���֮��Ϊ��������| A�� | 6��3��2 | B�� | 1��2��3 | C�� | 3��2��1 | D�� | 4��3��2 |
| A�� | Ԫ�����ڱ��У���18�����м���18���� | |
| B�� | ����������Ϊ2��Ԫ��ԭ�ӣ���һ�����ڢ�A��Ԫ�� | |
| C�� | �����ڱ��Ԫ�����ڵ�������������ԭ�Ӻ���ĵ��Ӳ��� | |
| D�� | ���������Ԫ�ض��ǽ���Ԫ�� |

 ��
��