��Ŀ����
12�����ܵĴ洢������Ӧ�õ���Ҫƿ����Ŀǰ�����û������о�����Ҫ��������У���λ�⻯��������廯���̼�ʲ��ϡ������⻯��ȣ���1��Ti��BH4��2��һ�ֹ���Ԫ�����⻯�ﴢ����ϣ�
��Ti2+��̬�ĵ����Ų�ʽ�ɱ�ʾΪ1s22s22p63s23p63d2��[Ar]3d2��
��BH4-�Ŀռ乹�����������壨��������������
��Ti����B��C��N��O�ȷǽ���Ԫ���γ��ȶ��Ļ�����縺��C��B�������ͬ������һ�����ܣ�N��O��ԭ����N ��p���Ϊ�����״̬����O��״̬���ȶ�
��2��Һ���Ǹ������ʣ������ܵ��������壬����N2+3H2?2NH3ʵ�ִ�������⣮����˵����ȷ����ABC������ѡ��
A�� NH3������Nԭ�Ӳ���sp3�ӻ�
B�� ��ͬѹǿʱ��NH3�е��PH3��
C��[Cu ��NH3��4]2+�����У�Nԭ������λԭ��
D��CN-�ĵ���ʽΪ[•C����N•]-
��3��2008�꣬Yoon���˷���Ca��C60�����ӽṹ��ͼ1�����ɵ�Ca32C60�ܴ�������H2���ӣ�
��C60���������ڱ���CS2��C60�ǷǼ��Է��ӣ�����ԡ��Ǽ��ԡ�����
��1mol C60�����У����ЦҼ���ĿΪ90NA��
��4��MgH2�ǽ����⻯�ﴢ����ϣ��侧���ṹ��ͼ2��ʾ����֪�þ�����ܶ�Ϊa g•cm-3���������Ϊ$\frac{52}{a{N}_{A}}$cm3[a��NA��ʾ��NA��ʾ�����ӵ�������ֵ��]��

���� ��1��������22��Ԫ�أ�Ti2+������20�����ӣ����ݹ���ԭ����д���̬��������Ų�ʽ��
�ڸ��ݼ۲���ӶԻ�������ȷ���ռ乹�ͣ�
��ͬһ����Ԫ�أ�Ԫ�صĵ縺������ԭ�����������������Ԫ�صĵ�һ����������ԭ�����������������������ƣ�����IIA��͵�VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�ԭ�ӵĹ���е��Ӵ��ڰ�����ȫ����ȫ��ʱ�Ƚ��ȶ���
��2��a�����ݼ۲���ӶԻ�������ȷ���ӻ���ʽ��
b��ͬһ����Ԫ�ص��⻯���У�����������⻯��е�ϸߣ�
c���ṩ�µ��ӶԵ�ԭ������ԭ�ӣ�
d��CN-�Ľṹ�͵����������ƣ����ݵ������ӵĵ���ʽ�жϣ�
��3���ٸ�����������ԭ��ȷ�����ӵļ��ԣ�
�����þ�̯�����㣻
��4�����þ�̯������þ�����þ����ԭ�Ӹ������ٸ���V=$\frac{m}{��}$���м��㣮
��� �⣺��1��������22��Ԫ�أ�Ti2+������20�����ӣ����ݹ���ԭ��֪���̬��������Ų�ʽΪ��1s22s22p63s23p63d2��[Ar]3d2���ʴ�Ϊ��1s22s22p63s23p63d2��[Ar]3d2��
��BH4-��Bԭ�Ӽ۲���Ӷ�=4+$\frac{1}{2}$����û�йµ��Ӷԣ���������������ṹ���ʴ�Ϊ���������壻
��C��B����ͬһ������C��ԭ����������B�����Ե縺��C��B��Nԭ����2p������ڰ����״̬��Oԭ����2p����Ȳ��ǰ����Ҳ����ȫ�ջ�ȫ��������Nԭ�ӵ�һ�����ܱ�Oԭ�Ӵ�
�ʴ�Ϊ����������N ��p���Ϊ�����״̬����O��״̬���ȶ���
��2��A��NH3������Nԭ�Ӻ���3�����õ��ӶԺ�һ���µ��Ӷԣ�������۲���Ӷ���4������sp3�ӻ�������ȷ��
B����ͬѹǿʱ�������к��������PH3�в������������NH3�е��PH3�ߣ�����ȷ��
C��[Cu��NH3��4]2+�����У�Nԭ���ṩ�µ��Ӷԣ�����Nԭ������λԭ�ӣ�����ȷ��
D��CN-�ĵ���ʽΪ ���ʴ���
���ʴ���
��ѡABC��
��3���ٱ���CS2���ǷǼ��Է��ӣ�������������ԭ��֪��C60�ǷǼ��Է��ӣ��ʴ�Ϊ���Ǽ��ԣ�
�����þ�̯��֪��ÿ��̼ԭ�Ӻ���$\frac{1}{2}$���� ��������1mol C60�����У����Ц� ����Ŀ=$\frac{3}{2}$��1mol��60��NA/mol=90NA���ʴ�Ϊ��90NA��
��4���þ�����þԭ�Ӹ���=$\frac{1}{8}$��ԭ�Ӹ���=2+4��$\frac{1}{2}$��V=$\frac{m}{��}$=$\frac{\frac{M}{{N}_{A}}��24��2+1��4��}{a}$cm3=$\frac{52}{a{N}_{A}}$cm3���ʴ�Ϊ��$\frac{52}{a{N}_{A}}$��
���� ���⿼�����ʽṹ�����ʣ��漰��������Ų����ӻ���ʽ���жϡ��ռ乹�͵��жϡ������ļ����֪ʶ�㣬�ѵ��Ǿ����ļ��㣬������ù�ʽ�ǽⱾ��ؼ����Ѷ��еȣ�

| A�� | Na2CO3��BaCl2 | B�� | HCl��KNO3 | C�� | HCl��Na2CO3 | D�� | Na2CO3��KNO3 |
| A�� | 15 | B�� | 23 | C�� | 28 | D�� | 32 |
| A�� | 2mol A+1mol B | |
| B�� | 1mol C+1mol D | |
| C�� | 2mol C+2mol D | |
| D�� | 0.5mol A+0.5mol B+0.5mol C+1mol D |
| A�� | WO3��WO2.9��ͬ���칹�� | |
| B�� | ��WO3�Ʊ�WO2.9�Ĺ��̷�����������ԭ��Ӧ | |
| C�� | 18g H2O��WO2.9�ĸ�Ч���²���H2���Ϊ22.4L | |
| D�� | �����������ʹ����ֽ�ˮ��ͬʱ�ɷų����� |
| A�� | �ƾ� | B�� | �������� | C�� | һˮ�ϰ� | D�� | ���ᱵ |
 ѧϰ��ѧӦ����ȷ����������������������ȥ���ĵ�����
ѧϰ��ѧӦ����ȷ����������������������ȥ���ĵ�����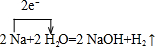 ��
��