��Ŀ����
20����������A��B��һ�������·������·�Ӧ��A+B��C+D+H2O��δ��ƽ��������ȥ��I����AΪһԪǿ�ᣨ���ᣩ
��1����BΪС�մ÷�Ӧ�Ļ�ѧ����ʽΪHCl+NaHCO3=NaCl+CO2��+H2O��
��2����C����ɫ���嵥�ʣ�д����Ӧ�Ļ�ѧ����ʽΪMnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O��
��3����C��D������Ԫ����ȫ��ͬ���÷�Ӧ�Ļ�ѧ����ʽΪFe3O4+8HCl=FeCl2+2FeCl3+4H2O��
II����AΪһԪǿ��������ƣ�
��4����C��ʹʪ��ĺ�ɫʯ����ֽ��������Ӧ�Ļ�ѧ����ʽΪNH4Cl+NaOH$\frac{\underline{\;\;��\;\;}}{\;}$NaCl+NH3��+H2O��
��5����B����ɫ���嵥�ʣ�������D��ˮ��Һ�ʼ��ԣ����շ�����SO2�����ӷ���ʽΪClO-+SO2+2OH-=Cl-+SO42-+H2O��
��6����B������Ԫ����ɵĵ��ʣ�C��D�������Σ��Ҿ���ʹ����KMnO4��Һ��ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ��3S+6NaOH$\frac{\underline{\;\;��\;\;}}{\;}$2Na2S+Na2SO3+3H2O��
���� I����AΪһԪǿ�ᣨ���ᣩ��
��1����BΪС�մ�������̼�����Ʒ�Ӧ�����Ȼ��ơ�������̼��ˮ��
��2����C����ɫ���嵥�ʣ�Ϊ����������Ũ���ᷴӦ�����Ȼ��̡�������ˮ��
��3����C��D������Ԫ����ȫ��ͬ�����������������ᷴӦ�����Ȼ��������Ȼ�����ˮ������ת����ϵ��
II����AΪһԪǿ��������ƣ�
��4����C��ʹʪ��ĺ�ɫʯ����ֽ�������Ȼ�����������Ʒ�Ӧ�����Ȼ��ơ�������ˮ����ת����ϵ��
��5����B����ɫ���嵥�ʣ���BΪ������������D��ˮ��Һ�ʼ��ԣ�����DΪNaClO��NaClO����Һ�н�������������Ϊ�����������ԭΪ�����ӣ�
��6����B������Ԫ����ɵĵ��ʣ�C��D�������Σ��Ҿ���ʹ����KMnO4��Һ��ɫ��Ϊ�����������Ʒ�Ӧ�������ơ�����������ˮ��
��� �⣺I����AΪһԪǿ�ᣨ���ᣩ��
��1����BΪС�մ�������̼�����Ʒ�Ӧ�����Ȼ��ơ�������̼��ˮ����Ӧ����ʽΪ��HCl+NaHCO3=NaCl+CO2��+H2O��
�ʴ�Ϊ��HCl+NaHCO3=NaCl+CO2��+H2O��
��2����C����ɫ���嵥�ʣ�Ϊ����������Ũ���ᷴӦ�����Ȼ��̡�������ˮ����Ӧ����ʽΪ��MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O��
�ʴ�Ϊ��MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O��
��3����C��D������Ԫ����ȫ��ͬ�����������������ᷴӦ�����Ȼ��������Ȼ�����ˮ������ת����ϵ����Ӧ����ʽΪ��Fe3O4+8HCl=FeCl2+2FeCl3+4H2O��
�ʴ�Ϊ��Fe3O4+8HCl=FeCl2+2FeCl3+4H2O��
II����AΪһԪǿ��������ƣ�
��4����C��ʹʪ��ĺ�ɫʯ����ֽ�������Ȼ�����������Ʒ�Ӧ�����Ȼ��ơ�������ˮ����ת����ϵ����Ӧ����ʽΪ��NH4Cl+NaOH$\frac{\underline{\;\;��\;\;}}{\;}$NaCl+NH3��+H2O��
�ʴ�Ϊ��NH4Cl+NaOH$\frac{\underline{\;\;��\;\;}}{\;}$NaCl+NH3��+H2O��
��5����B����ɫ���嵥�ʣ���BΪ������������D��ˮ��Һ�ʼ��ԣ�����DΪNaClO��NaClO����Һ�н�������������Ϊ�����������ԭΪ�����ӣ���Ӧ���ӷ���ʽΪ��ClO-+SO2+2OH-=Cl-+SO42-+H2O��
�ʴ�Ϊ��ClO-+SO2+2OH-=Cl-+SO42-+H2O��
��6����B������Ԫ����ɵĵ��ʣ�C��D�������Σ��Ҿ���ʹ����KMnO4��Һ��ɫ��Ϊ�����������Ʒ�Ӧ�������ơ�����������ˮ����Ӧ����ʽΪ��3S+6NaOH$\frac{\underline{\;\;��\;\;}}{\;}$2Na2S+Na2SO3+3H2O��
�ʴ�Ϊ��3S+6NaOH$\frac{\underline{\;\;��\;\;}}{\;}$2Na2S+Na2SO3+3H2O��
���� ���⿼�������ƶϣ���Ҫѧ����������Ԫ�ػ��������ʣ��ѶȲ���ּ�ڿ���ѧ���Ի���֪ʶ�����գ�

| A�� | ��Ӧ���������ͻ�ԭ����������Ϊ1��2 | |
| B�� | ��Ӧ��������8g Cu2S | |
| C�� | ��Ӧ����0.2��6.02��1023������ת�� | |
| D�� | ��Ӧ����1.6g������ |
| A�� | 5 | B�� | 6 | C�� | 7 | D�� | 8 |
| A�� | X��3s23p1 Y��3s23p5 | B�� | X��2s22p3 Y��2s22p4 | ||
| C�� | X��3s23p1 Y��2s22p4 | D�� | X��3s2 Y��2s22p3 |
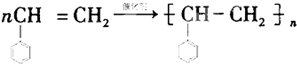
 B����ϩ
B����ϩ
 2NH3
2NH3 ��ͼΪ����ʵ��װ�ã�
��ͼΪ����ʵ��װ�ã�
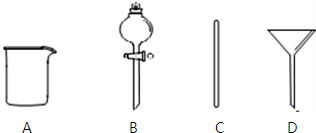 ʵ������Ҫ0.1mol•L-1NaOH��Һ480mL��
ʵ������Ҫ0.1mol•L-1NaOH��Һ480mL��


 ��
�� ��
�� ��
�� ��
�� ��
��