��Ŀ����
4����ͼ��ʾһЩ�����е�ijЩ�ṹ�����Ƿֱ���NaCl��CsCl���ɱ������ʯ��ʯī����ṹ�е�ijһ�ֵ�ijһ���֣�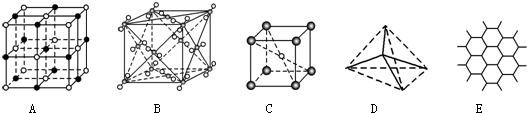
��1���������ʯ����D������ĸ��ţ���ͬ��������ÿ��̼ԭ����4��̼ԭ������Ҿ�����ȣ����ʯ����ԭ�Ӿ��壮
��2������ʯī����E��ÿ����������ռ�е�̼ԭ����ƽ��Ϊ2����
��3������NaCl����A��ÿ��Na+��Χ��������Ҿ�����ȵ�Na+��12����
��4������CsCl����C�����������Ӿ��壬ÿ��Cs+���8Cl-���ڣ�
��5�������ɱ�����B�������ڷ��Ӿ��壬ÿ��CO2������12��CO2���ӽ��ڣ�
��6�����������������۵��ɸߵ��͵�����˳��Ϊʯī�����ʯ��NaCl��CsCl���ɱ�������ĸ��Żش𣩣�
���� ��1�����ʯ�ǿռ���״�ṹ��ÿ��̼ԭ������4��̼ԭ�ӣ�ԭ�Ӽ��Թ��ۼ����ϣ��ݴ˴��⣻
��2��ʯī�Dz�״�ṹ���ڲ����֮���Է��»�������ã��ڲ���̼��̼�Թ��ۼ�����ã��γ������Σ��ݴ˴��⣻
��3����NaCl�����У�ÿ����������Χ�����������ӣ�ÿ����������ΧҲ�����������ӣ����ݾ����Ľṹ��ÿ����������Χ�����������������С���������Խ��ߵ�λ�ã�ÿ����������Χ�а˸������������壬������Խ����ϵ������Ӿ���12�����ݴ˴��⣻
��4��CsCl�ľ���������Ӻ������ӵ���λ������8����ÿ���������Χ��8�������ӣ�ÿ����������ΧҲ��8������ӣ��ݴ˴��⣻
��5���ɱ��Ƿ��Ӿ��壬CO2����λ��������Ķ���������ϣ��Զ����ϵ�CO2����Ϊ�����������������CO2���ӷֲ�����ö���������12����������ϣ���6�����ݸ������ʵ��۵㣺ԭ�Ӿ��壾���Ӿ��壾���Ӿ����Լ������Ӿ�����뾶Խ������ԽС���۵�Խ�ͽ��
��� �⣺��1���ڽ��ʯ�����У�ÿ��̼������Χ4��̼ԭ���γɹ��ۼ�������4��̼ԭ����������������γ��������壬������һ��̼ԭ�ӣ�����ͼDΪ���ʯ��ÿ��̼ԭ����4��̼ԭ������Ҿ�����ȣ����ʯ�ǿռ���״�ṹ������ԭ�Ӿ��壻
�ʴ�Ϊ��D��4��ԭ�ӣ�
��2��ʯī�Dz�״�ṹ���ڲ����֮���Է��»�������ã��ڲ���̼��̼�Թ��ۼ�����ã��γ������Σ�����ͼEΪʯī�Ľṹ��ÿ����������ռ�е�̼ԭ����ƽ��Ϊ6��$\frac{1}{3}$=2��
�ʴ�Ϊ��E��2��
��3����NaCl�����У�ÿ����������Χ�����������ӣ�ÿ����������ΧҲ�����������ӣ�����ͼAΪNaCl�Ľṹ�����ݾ����Ľṹ��ÿ����������Χ�����������������С���������Խ��ߵ�λ�ã�ÿ����������Χ�а˸������������壬����ÿ����������Χ��������Ҿ�����ȵ������Ӿ���12����
�ʴ�Ϊ��A��12��
��4��CsCl�ľ���������Ӻ������ӵ���λ������8����ÿ���������Χ��8�������ӣ�ÿ����������ΧҲ��8������ӣ�����ͼCΪCsCl�Ľṹ���������Ӿ��壻
�ʴ�Ϊ��C�����ӣ�8��
��5���ɱ��Ƿ��Ӿ��壬CO2����λ��������Ķ���������ϣ��Զ����ϵ�CO2����Ϊ�����������������CO2���ӷֲ�����ö���������12����������ϣ�����ͼBΪ�ɱ����壻
�ʴ�Ϊ��B�����ӣ�12��
��6���������ʵ��۵㣺ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壬ʯī���۵���ڽ��ʯ�������Ӿ�����뾶Խ������ԽС���۵�Խ�ͣ���������Ӱ뾶���������ӣ�ʯī���۵���ڽ��ʯ�������۵��ɸߵ��͵�����˳��Ϊ��ʯī�����ʯ��NaCl��CsCl���ɱ���
�ʴ�Ϊ��ʯī�����ʯ��NaCl��CsCl���ɱ���
���� ������Ҫ�����˸��ֳ������͵ľ���Ľṹ���Լ����־�������ʣ��ѶȲ�����Ĺؼ���Ҫ�Ի���֪ʶҪ��������ǽṹͼ��

| A�� | ʯ�͡�ú����Ȼ�������ڻ�ʯȼ�� | |
| B�� | �Է���Ӧһ�����������Է���Ӧһ�����ؼ�С | |
| C�� | ������ˮΪ֮��������ˮ��˵����ͬ������ˮ�ͱ������������� | |
| D�� | SiO2��������NaOH��Һ��������HF��Һ��˵��SiO2������������ |
 ���⾫��ѧ������������ޣ���ҩ��Ȳ�����ҩ��Ҳ�����������Ӽ������������������˷ܼ����������Ӷ��������������������ʹ�ã�ʹ��Ʒ�������У����ͳɱ��������������������ã�������������ӽṹ��ʽ��ͼ������˵������ȷ���ǣ�������
���⾫��ѧ������������ޣ���ҩ��Ȳ�����ҩ��Ҳ�����������Ӽ������������������˷ܼ����������Ӷ��������������������ʹ�ã�ʹ��Ʒ�������У����ͳɱ��������������������ã�������������ӽṹ��ʽ��ͼ������˵������ȷ���ǣ�������| A�� | ���⾫�ķ���ʽ��C11H18ON2Cl2 | |
| B�� | �����ܷ���ȡ����Ӧ��������Ӧ���ӳɷ�Ӧ��ˮ�ⷴӦ | |
| C�� | 1mol������������������4molNaOH��Ӧ | |
| D�� | ���������������������7��̼ԭ�ӹ��� |
| A�� | HCl��HBr��HI���ۡ��е�������������Ӽ���������С�й� | |
| B�� | H2O���ۡ��е����H2S������H2O����֮�������� | |
| C�� | I2������CCl4��������������ԭ������ | |
| D�� | �������ˮ�γ�������ֻ�ѧ�� |
| A�� | ��̬�⻯����ȶ���X��Y��Z��˳����� | |
| B�� | ����Ԫ�ص�����������Ӧ��ˮ�������ԣ�H2ZO3��H3YO4��H2XO4 | |
| C�� | Ԫ�ص���������ϼ۰�X��Y��Z��˳��ݼ� | |
| D�� | Ԫ��ԭ�ӵİ뾶��X��Y��Z��˳��ݼ� |

| A�� | ʯī�缫��ֱ����Դ�������� | |
| B�� | ͭ�缫�ķ�ӦʽΪ��2H++2e-�TH2�� | |
| C�� | ����������ʯī�缫����������Na+��ʯī�缫Ǩ�� | |
| D�� | ��ʪ��KI�����Լ���ͭ�缫�����������壬��ֽ����ɫ |
| A�� | H2SO4��CuSO4 | B�� | MnO4-��Mn2+ | C�� | Fe��FeCl3 | D�� | NaNO3��N2 |
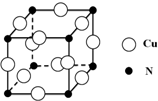 ��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺
��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺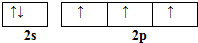 ��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3��
��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3��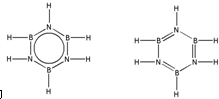 ��
�� Ϊ�������ˮ��������������ͼ��ʾ��ʵ�飬�Թܼͱ�����60��80���ˮԡ����5��6min���Թ��Ҳ����ȣ����Թܼ��е���Һ��ȴ���ٽ��к���ʵ�飮
Ϊ�������ˮ��������������ͼ��ʾ��ʵ�飬�Թܼͱ�����60��80���ˮԡ����5��6min���Թ��Ҳ����ȣ����Թܼ��е���Һ��ȴ���ٽ��к���ʵ�飮