��Ŀ����
18�� ijѧ����0.1000 mol•L-1KOHҺ�ζ�δ֪Ũ�ȵĴ��ᣬ������ֽ�Ϊ���¼�����
ijѧ����0.1000 mol•L-1KOHҺ�ζ�δ֪Ũ�ȵĴ��ᣬ������ֽ�Ϊ���¼�����A����ȡ20mL�������ע��ྻ����ƿ��������2��3��ָʾ��
B���ñ���Һ��ϴ�ζ���2��3��
C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ��KOH���ʽ�ζ�������0���̶�����1��2cm
E������Һ������0����0������ijһ�̶ȣ����¶���
F������ƿ���ڵζ��ܵ����棬�ñ�KOH��Һ�ζ����յ㲢���µζ���Һ��Ŀ�
�ȣ�
�ʹ�ʵ�������գ�
��1����ȷ���������˳���ǣ��������ĸ��д��BDCEAF��
��2������B���������Ŀ���Ƿ�ֹ�ζ����ڱڸ��ŵ�ˮ������Һϡ�Ͷ�������
��3������A�������֮ǰ�����ô���Һ��ϴ��ƿ��������յζ������Ӱ����
�������������С�������䡱��
��4��A�����У�ʹ�õ�ָʾ��Ϊ��̪��Һ���жϵ���ζ��յ��ʵ�������ǵ��������һ����Һʱ����ƿ����Һ����ɫ��Ϊdz��ɫ���Ұ���Ӳ���ɫ��
��5������ͼ1Ϊijһ�μ�ʽ�ζ��ܵζ�ǰҺ�棬�����ֵΪ0.70mLͼ2Ϊ�ζ�����Һ�棬�εζ����̹�ʹ����20.00mLKOH����Һ
��6��Ϊ�궨ij������Һ��ȷŨ�ȣ���0.1000mol•L-1��NaOH��Һ��20.00mL������Һ���еζ������εζ�����NaOH��Һ��������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����NaOH��Һ�������mL�� | 20.05 | 20.00 | 18.80 | 19.95 |
���� ��1�������к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ��Ȳ�����
��2���ñ�NaOH��Һ��ϴ�ζ��ܣ���ֹ������
��3������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��4������KOH�ʹ��ᷴӦ���ɴ���أ���Һ�ʼ��ԣ�ѡ�÷�̪��Һ��ָʾ��������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��5�����ݵζ��ܵĽṹ�������Լ�����ԭ�������
��6���ȸ������ݵ���Ч�ԣ���ȥ��3�����ݣ�Ȼ�����1��2��4��ƽ������V��NaOH�������Ÿ���CH3COOH+NaOH=CH3COONa+H2O���c��CH3COOH����
��� �⣺��1���к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ���˳�����������ȷ��˳��Ϊ��BDCEAF��
�ʴ�Ϊ��BDCEAF��
��2���ñ�NaOH��Һ��ϴ�ζ���2��3�Σ���ֹ�ζ����ڱڸ��ŵ�ˮ������Һϡ�Ͷ�������
�ʴ�Ϊ����ֹ�ζ����ڱڸ��ŵ�ˮ������Һϡ�Ͷ�������
��3����ƿ������ˮϴ�Ӻ�������ô���Һ��ϴ����ʹ��ƿ�����ʵ����ʵ����������V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$������c�����⣩����
�ʴ�Ϊ������
��4��KOH�ʹ��ᷴӦ���ɴ���أ���Һ�ʼ��ԣ�ѡ�÷�̪��Һ��ָʾ������ʵ������KOH�ζ����ᣬ�÷�̪��ָʾ���������յ��ǣ����������һ����Һʱ����ƿ����Һ����ɫ��Ϊdz��ɫ���Ұ���Ӳ���ɫ��
�ʴ�Ϊ����̪��Һ�����������һ����Һʱ����ƿ����Һ����ɫ��Ϊdz��ɫ���Ұ���Ӳ���ɫ��
��5����������ͼ�ϵĿ̶ȿ�֪���ζ�ǰ��ֵΪ0.70mL���ζ�����ֵΪ20.70mL������KOH����Һ�����Ϊ20.00mL��
�ʴ�Ϊ��0.70��20.00��
��6���Ĵ����ĵĴ��������ֱ�Ϊ 20.05ml��20.00ml��18.80ml��19.95ml������������������Ӧ����ȥ���������ε�ƽ��ֵΪ20.00ml��
��CH3COOH+NaOH=CH3COONa+H2O�ã�n��CH3COOH��=n��NaOH������C��CH3COOH����0.02L=0.1000mol•L-1��0.02L��
���c��CH3COOH��=$\frac{0.1000mol•{L}^{-1}��0.02L}{0.02L}$=0.1000mol•L-1
�ʴ�Ϊ��0.1000mol•L-1��
���� ������Ҫ�������к͵ζ��������������Լ����㣬�������ʱҪ���Ƿ�Ӱ��V���������ɣ��ѶȲ���

| A�� | 0.1 mol•L-1 | |
| B�� | һ������0.1 mol•L-1 | |
| C�� | ��Ϊǿ��һ������0.1 mol•L-1����Ϊ����һ��С��0.1 mol•L-1 | |
| D�� | ��Ϊǿ��һ����0.1 mol•L-1����Ϊ����һ����0.1 mol•L-1 |
| A�� | 0.05mol•L-1��H2SO4 | B�� | 0.1mol•L-1��KNO3 | ||
| C�� | 0.1mol•L-1��KOH | D�� | 0.1mol•L-1��NH4NO3 |
| A�� | ��ƿϴ������������ˮ | |
| B�� | ȡ��Һ�ĵζ��ܿ�ʼ�����ݣ��ų�Һ���������ʧ | |
| C�� | ��Һ��ɫ��dzʱ������Һ���죬ֹͣ�ζ���һ��NaOH��Һ�ޱ仯 | |
| D�� | �ü�ʽ�ζ���ȡһ�������NaOH��Һʱ����ȡǰ���Ӷ�������ȡ���Ӷ��� |
 ��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����| CH3COOH | HClO | H2CO3 |
| Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.4��10-7Ka2=4.7��10-11 |
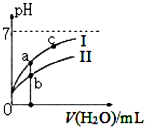
| A�� | ͼ��c��H+����c��R-����ֵ��a�㣾c�㣨HR����CH3COOH��HCIO�� | |
| B�� | pH��ͬ��������ҺŨ�ȹ�ϵ��c��CH3COONa��c��NaHC03��c��NaClO��c��Na2C03�� | |
| C�� | ͼ��a�������Ũ��С��b�������Ũ�� | |
| D�� | Ũ�Ⱦ�Ϊ0��l mol/L��CH3COONa��NaCIO�Ļ����Һ�У�c��OH-��=0��l mol/L-c��ClO-��+c��H+��+c��CH3COOH�� |
| A�� | �������ˮ���Ʊ������ɱ�ϩ��ˮ��Ӧ�Ʊ��� | |
| B�� | �ɱ������������������Ҵ���������ȩ | |
| C�� | ��������Ҵ�����������������������ˮ�����Ҵ� | |
| D�� | ���ȴ���������ϩ���ɱ�ϩ��1��2-������� |
| A�� | 2-��-4-�һ����� | B�� | 3��4-���һ�-4-��ϩ | ||
| C�� | 2-��-3-��Ȳ | D�� | �Զ��ױ� |