��Ŀ����
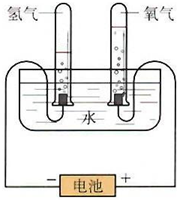 ijѧϰС�����ͼ����ʾ�ĵ��ˮԭ������ʵ�飬����ʵ��õ�����������ϻ�õ����ݽ��д�������������������һ�����̽�����밴Ҫ����д��
ijѧϰС�����ͼ����ʾ�ĵ��ˮԭ������ʵ�飬����ʵ��õ�����������ϻ�õ����ݽ��д�������������������һ�����̽�����밴Ҫ����д����1���۲첻ͬʱ���Թ��ڵ�H2��O2����ı�ֵ��ԼΪ
��2����������1.8g H2O�����ݵ��ˮ�Ļ�ѧ����ʽ��������H2��O2��������������£�
| ����g | ���ʵ���mol | H2 ��O2���ʵ����ı� | |
| H2 | |||
| O2 |
��3���±��г���һЩ���ʵ����
| ���� | ���� | 1mol���ʵ���� |
| 0��101kPa | H2 | 22.3L |
| O2 | 22.4L | |
| CO2 | 22.4L | |
| 25��101kPa | H2 | 24.4L |
| O2 | 24.5L | |
| CO2 | 24.5L |
������ͬ���¶�ѹǿ�£�
��
��
��4������۵ĽǶȽ�����������仯���ɵ�ԭ��
���㣺�����ӵ����ɼ�����
ר�⣺�����ӵ������Ͱ����ӵ�����
��������1������ʽΪ2H2O
=2H2��+O2����H2��O2����ı�ֵ�������ʵ���֮�ȣ�
��2����Ϸ�Ӧ�ķ���ʽ���㣻
��3������Ħ��������¶ȡ�ѹǿ�йأ�����ͬ���¶ȡ�ѹǿ�����£������Ħ�������ȣ�
��4���������Ӽ�ľ���ԶԶ�������ӱ�����ֱ��������ͬ���¶�ѹǿ�£��κ��������Ӽ�ľ�����ͬ��
| ||
��2����Ϸ�Ӧ�ķ���ʽ���㣻
��3������Ħ��������¶ȡ�ѹǿ�йأ�����ͬ���¶ȡ�ѹǿ�����£������Ħ�������ȣ�
��4���������Ӽ�ľ���ԶԶ�������ӱ�����ֱ��������ͬ���¶�ѹǿ�£��κ��������Ӽ�ľ�����ͬ��
���
�⣺��1������ʽΪ2H2O
=2H2��+O2����H2��O2����ı�ֵ�������ʵ���֮�ȣ�Ϊ2��1���ʴ�Ϊ��2��1��
��2��2H2O
2H2��+O2����
36 4 32
8g 0.2 g 1.6 g
n��H2��=
=0.1mol��n��O2��=
=0.05mol��
H2 ��O2���ʵ����ı�2��1��
���ݸ�ʵ��Ĺ۲�����������õ���������������ɵ�һ�������ǣ�����ͬ���¶Ⱥ�ѹǿ�£���ͬ������κ����嶼������ͬ��Ŀ�ķ��ӣ�ͬ��ͬѹ�£���������֮�ȵ��������ʵ���֮�ȣ�Ҳ�����������֮�ȣ�
�ʴ�Ϊ��
ͬ��ͬѹ�£���ͬ������κ����嶼������ͬ��Ŀ�ķ��ӣ�ͬ��ͬѹ�£���������֮�ȵ��������ʵ���֮�ȣ�Ҳ�����������֮�ȣ�
��3���ɱ������ݿɵã�������ͬ���¶�ѹǿ�£���ͬ��Ŀ�ķ��������κ����嶼������ͬ�����
������ͬ���¶�ѹǿ�£�1Ħ����ͬ����������һ���ģ�
������ͬ��ѹǿ�£������¶ȵ����ߣ�1Ħ��������������
�ʴ�Ϊ��������ͬ���¶�ѹǿ�£���ͬ��Ŀ�ķ��������κ����嶼������ͬ�����
������ͬ���¶�ѹǿ�£�1Ħ����ͬ����������һ���ģ�
������ͬ��ѹǿ�£������¶ȵ����ߣ�1Ħ��������������
��4����ʵ���֪���������Ӽ�ľ���ԶԶ�������ӱ�����ֱ��������ͬ���¶�ѹǿ�£��κ��������Ӽ�ľ�����ͬ����ˣ�������Ŀ��ͬ���κ����������ͬ�������
�ʴ�Ϊ���������Ӽ�ľ���ԶԶ�������ӱ�����ֱ��������ͬ���¶�ѹǿ�£��κ��������Ӽ�ľ�����ͬ����ˣ�������Ŀ��ͬ���κ����������ͬ�������
| ||
��2��2H2O
| ||
36 4 32
8g 0.2 g 1.6 g
n��H2��=
| 0.2g |
| 2g/mol |
| 1.6g |
| 32g/mol |
H2 ��O2���ʵ����ı�2��1��
���ݸ�ʵ��Ĺ۲�����������õ���������������ɵ�һ�������ǣ�����ͬ���¶Ⱥ�ѹǿ�£���ͬ������κ����嶼������ͬ��Ŀ�ķ��ӣ�ͬ��ͬѹ�£���������֮�ȵ��������ʵ���֮�ȣ�Ҳ�����������֮�ȣ�
�ʴ�Ϊ��
| ����g | ���ʵ���mol | H2 ��O2���ʵ����ı� | |
| H2 | 0.2 | 0.1 | 2��1 |
| O2 | 1.6 | 0.05 |
��3���ɱ������ݿɵã�������ͬ���¶�ѹǿ�£���ͬ��Ŀ�ķ��������κ����嶼������ͬ�����
������ͬ���¶�ѹǿ�£�1Ħ����ͬ����������һ���ģ�
������ͬ��ѹǿ�£������¶ȵ����ߣ�1Ħ��������������
�ʴ�Ϊ��������ͬ���¶�ѹǿ�£���ͬ��Ŀ�ķ��������κ����嶼������ͬ�����
������ͬ���¶�ѹǿ�£�1Ħ����ͬ����������һ���ģ�
������ͬ��ѹǿ�£������¶ȵ����ߣ�1Ħ��������������
��4����ʵ���֪���������Ӽ�ľ���ԶԶ�������ӱ�����ֱ��������ͬ���¶�ѹǿ�£��κ��������Ӽ�ľ�����ͬ����ˣ�������Ŀ��ͬ���κ����������ͬ�������
�ʴ�Ϊ���������Ӽ�ľ���ԶԶ�������ӱ�����ֱ��������ͬ���¶�ѹǿ�£��κ��������Ӽ�ľ�����ͬ����ˣ�������Ŀ��ͬ���κ����������ͬ�������
�����������ۺϿ��鰢��٤�����ɵIJⶨ��������ѧ���ķ���������ʵ�������ͼ��������Ŀ��飬Ϊ��Ƶ���㣬ע�����ݵĶԱȺ���Ϣ�����ϣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
���з�Ӧ������������ԭ��Ӧ���ǣ�������
| A��NaClO+CH3COOH�TCH3COONa+HClO | ||||
B��Cu2��OH��2CO3
| ||||
| C��2NO2+2NaOH�TNaNO3+NaNO2+H2O | ||||
| D��3CCl4+K2Cr2O7�T2CrO2Cl2+3COCl2+2KCl |
��NAΪ�����ӵ�����������˵����ȷ���ǣ�������
| A����������H2��Cl2�����ķ�����һ���� |
| B��2mol?L-1��MgCl2��Һ�к�Mg2+��Ϊ2NA |
| C�����³�ѹ�£�11.2LCO2����������Ϊ0.5NA |
| D�������£�8g���飨CH4����������ԭ����Ϊ2NA |
���н���ʵ����ʵ�����ӷ���ʽ����ȷ���ǣ�������
| A��Fe��ϡH2SO4��Ӧ�ų����壺Fe+6H+=2Fe3++3H2�� |
| B��ϡH2SO4��BaCl2��Һ��Ӧ���ɳ�����Ba2++SO42-�TBaSO4�� |
| C���ô���ʯ��ϡ�����Ʊ�CO2��CaCO3+2H+=Ca2++CO2��+H2O |
| D��̼������Һ������ϡ���CO32-+2H+=CO2��+H2O |
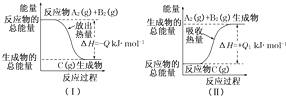
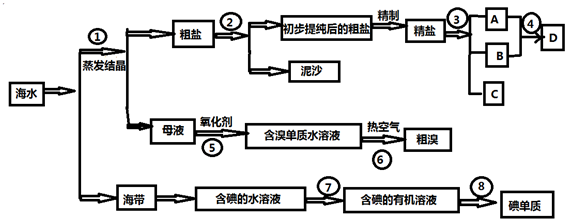
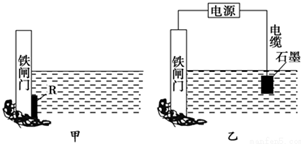
 ����������������ȼ���������ֺϳɹ�����ͼ��ʾ��
����������������ȼ���������ֺϳɹ�����ͼ��ʾ��