��Ŀ����
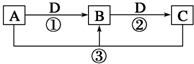 ��֪A��B��C��D����ѧ��ѧ�ij������ʣ���A��B��C������ͬһ��Ԫ�أ���һ������������֮����ת����ϵ��ͼ��ʾ�����ַ�Ӧ�е�H2O����ȥ����
��֪A��B��C��D����ѧ��ѧ�ij������ʣ���A��B��C������ͬһ��Ԫ�أ���һ������������֮����ת����ϵ��ͼ��ʾ�����ַ�Ӧ�е�H2O����ȥ������ش��������⣺
��1����A����������ˮ������D�����������������������;���Ľ������ʣ���������B����Һ���ܵõ�B����B�Ļ�ѧʽ������
��2����A��һ�ּ������壬�������������B������β��֮һ�����������ɫ����Ӧ�ٵĻ�ѧ����ʽΪ
��3����D���ȼҵ����Ҫ��Ʒ֮һ��B�����ԣ���Ӧ�ڵ����ӷ���ʽ��
��4����A��C��D���dz������壬C�ǵ����������Ҫ���壬��Ӧ�۵Ļ�ѧ����ʽΪ
���㣺������ƶ�
ר�⣺�ƶ���
��������1����A����������ˮ������ӦΪCl2��D�����������������������;���Ľ������ʣ�ӦΪFe����BΪFeCl3��CΪFeCl2��
��2����A��һ�ּ������壬�������������ӦΪNH3��B������β��֮һ�����������ɫ��ӦΪNO��DΪ������
��3����D���ȼҵ����Ҫ��Ʒ��B�����ԣ���BΪAl��OH��3����ת����ϵ��֪��DΪNaOH��CΪNaAlO2��
��4����A��C��D���dz������壬C���γ��������Ҫ���壬ӦΪSO2����AΪH2S��DΪO2��BΪS��
��2����A��һ�ּ������壬�������������ӦΪNH3��B������β��֮һ�����������ɫ��ӦΪNO��DΪ������
��3����D���ȼҵ����Ҫ��Ʒ��B�����ԣ���BΪAl��OH��3����ת����ϵ��֪��DΪNaOH��CΪNaAlO2��
��4����A��C��D���dz������壬C���γ��������Ҫ���壬ӦΪSO2����AΪH2S��DΪO2��BΪS��
���
�⣺��1����A����������ˮ������ӦΪCl2��D�����������������������;���Ľ������ʣ�ӦΪFe����BΪFeCl3��CΪFeCl2���ʴ�Ϊ��FeCl3��
��2����A��һ�ּ������壬�������������ӦΪNH3��B������β��֮һ�����������ɫ��ӦΪNO��DΪ��������Ӧ�ٵĻ�ѧ����ʽΪ��4NH3+5O2
4NO+6H2O���ʴ�Ϊ��4NH3+5O2
4NO+6H2O��
��3����D���ȼҵ����Ҫ��Ʒ��B�����ԣ���BΪAl��OH��3����ת����ϵ��֪��DΪNaOH��CΪNaAlO2����Ӧ�ڵ����ӷ���ʽ�ǣ�Al��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��4����A��C��D���dz������壬C���γ��������Ҫ���壬ӦΪSO2����AΪH2S��DΪO2��BΪS����Ӧ�۵Ļ�ѧ����ʽΪ��2H2S+SO2�T3S��+2H2O���ʴ�Ϊ��2H2S+SO2�T3S��+2H2O��
��2����A��һ�ּ������壬�������������ӦΪNH3��B������β��֮һ�����������ɫ��ӦΪNO��DΪ��������Ӧ�ٵĻ�ѧ����ʽΪ��4NH3+5O2
| ||
| �� |
| ||
| �� |
��3����D���ȼҵ����Ҫ��Ʒ��B�����ԣ���BΪAl��OH��3����ת����ϵ��֪��DΪNaOH��CΪNaAlO2����Ӧ�ڵ����ӷ���ʽ�ǣ�Al��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��4����A��C��D���dz������壬C���γ��������Ҫ���壬ӦΪSO2����AΪH2S��DΪO2��BΪS����Ӧ�۵Ļ�ѧ����ʽΪ��2H2S+SO2�T3S��+2H2O���ʴ�Ϊ��2H2S+SO2�T3S��+2H2O��
���������⿼�������ƶϣ����ڿ�������Ŀ������һ���Ѷȣ���Ҫѧ����������Ԫ�ػ��������ʣ�

��ϰ��ϵ�д�
�����Ŀ
ˮ�ĵ������ΪH2O?H++OH-���ڲ�ͬ�¶�����ƽ�ⳣ��ΪKW��25�棩=1.0��10-14��K��35�棩=2.1��10-14����������ȷ���ǣ�������
| A��c��H+�������¶ȵ����߶����� |
| B��35��ʱ��ˮ��c��H+����c��OH-�� |
| C����ˮ�м���NaHSO4��Һ������ˮ�ĵ��� |
| D����ˮ�м���NaHCO3��Һ������ˮ�ĵ��� |
����������ȷ���ǣ���NA���������ӵ�������ֵ����������
| A������NA����ԭ�ӵĺ����ڱ�״���µ����ԼΪ11.2L |
| B��25��1.01��105Pa?64��SO2�к��е�ԭ����Ϊ3NA |
| C��22.4LCH4��18gH2O�����еĵ�������Ϊ10NA |
| D��CO��N2���Ӻ��еĵ�������ͬ��22.4L��CO������lmol N2�����ĵ�������� |
2g AO32-�к������������������3.01��1022������Ԫ��A��Ħ�������ǣ�������
| A��12g/mol |
| B��32g |
| C��80g/mol |
| D��32g/mol |