��Ŀ����
��1������FeCl3����Һʱ�������������ڽ�Ũ�� �У�Ȼ���ټ�ˮϡ�ͣ�
��2��������������ϴ���ۣ��Ҽ���Խǿȥ������Խǿ��������ʹ��Na2CO3ȥ����ʱ������ǽ�Na2CO3���� ˮ�У�
��3��������KAl��SO4��2?12H2O�����Ծ�ˮ��ԭ��������ˮ���������Ľ�״Al��OH��3��������ˮ������ʣ����γɳ�����ʹˮ���壬�����ӷ���ʽΪ ��
��4����ĭ������е�ҩƷΪNaHCO3��Al2��SO4��3������HCO3-��Al3+���ɷ���ˮ�⣬����ٽ�����������CO2�ͳ���Al��OH��3���䷴Ӧ�����ӷ���ʽΪ ��
��2��������������ϴ���ۣ��Ҽ���Խǿȥ������Խǿ��������ʹ��Na2CO3ȥ����ʱ������ǽ�Na2CO3����
��3��������KAl��SO4��2?12H2O�����Ծ�ˮ��ԭ��������ˮ���������Ľ�״Al��OH��3��������ˮ������ʣ����γɳ�����ʹˮ���壬�����ӷ���ʽΪ
��4����ĭ������е�ҩƷΪNaHCO3��Al2��SO4��3������HCO3-��Al3+���ɷ���ˮ�⣬����ٽ�����������CO2�ͳ���Al��OH��3���䷴Ӧ�����ӷ���ʽΪ
���㣺����ˮ���Ӧ��,���ӷ���ʽ����д
ר�⣺�����ˮ��ר��
��������1��FeCl3+3H2O?Fe��OH��3+3HCl��Ϊ����ˮ�⣬Ӧ�����Ȼ�����Һ�м�������HCl��
��2������ˮ�������ȷ�Ӧ�������¶ȴٽ�ˮ�⣻
��3�������к��������ӣ���������ˮ��ˮ�����������������壬������������Զ���ˮ��
��4��HCO3-��Al3+���ɷ���ˮ�⣬����ٽ�����������CO2�ͳ���Al��OH��3��
��2������ˮ�������ȷ�Ӧ�������¶ȴٽ�ˮ�⣻
��3�������к��������ӣ���������ˮ��ˮ�����������������壬������������Զ���ˮ��
��4��HCO3-��Al3+���ɷ���ˮ�⣬����ٽ�����������CO2�ͳ���Al��OH��3��
���
�⣺��1��FeCl3+3H2O?Fe��OH��3+3HCl��Ϊ����ˮ�⣬Ӧ�����Ȼ�����Һ�м�������HCl����������FeCl3����Һʱ�������������ڽ�Ũ�����У��ټ�ˮϡ�ͣ�
�ʴ�Ϊ���
��2������ˮ�������ȷ�Ӧ�������¶ȴٽ�ˮ�⣬̼������ˮ�������Һ�ʼ��ԣ�����Ϊ�˴ٽ�̼����ˮ�⣬Ӧ�ý�̼����������ˮ�У��ʴ�Ϊ���ȣ�
��3�������к��������ӣ���������ˮ��ˮ�����������������壬������������Զ���ˮ��ˮ�ⷽ��ʽΪAl3++3H2O?Al��OH��3�����壩+3H+���ʴ�Ϊ��Al3++3H2O?Al��OH��3�����壩+3H+��
��4��HCO3-��Al3+���ɷ���ˮ�⣬����ٽ�����������CO2�ͳ���Al��OH��3�����ӷ���ʽΪAl3++3HCO3-=Al��OH��3��+3CO2�����ʴ�Ϊ��Al3++3HCO3-=Al��OH��3��+3CO2����
�ʴ�Ϊ���
��2������ˮ�������ȷ�Ӧ�������¶ȴٽ�ˮ�⣬̼������ˮ�������Һ�ʼ��ԣ�����Ϊ�˴ٽ�̼����ˮ�⣬Ӧ�ý�̼����������ˮ�У��ʴ�Ϊ���ȣ�
��3�������к��������ӣ���������ˮ��ˮ�����������������壬������������Զ���ˮ��ˮ�ⷽ��ʽΪAl3++3H2O?Al��OH��3�����壩+3H+���ʴ�Ϊ��Al3++3H2O?Al��OH��3�����壩+3H+��
��4��HCO3-��Al3+���ɷ���ˮ�⣬����ٽ�����������CO2�ͳ���Al��OH��3�����ӷ���ʽΪAl3++3HCO3-=Al��OH��3��+3CO2�����ʴ�Ϊ��Al3++3HCO3-=Al��OH��3��+3CO2����
���������⿼��������ˮ�⣬��ȷ����ˮ��ԭ���ǽⱾ��ؼ���ע���Ԫ��������ӺͶ�Ԫ��������ˮ�����������Ӻ�̼�������������˫ˮ������ܹ��棬�������������ӹ����ѡ���У�Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
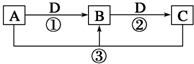 ��֪A��B��C��D����ѧ��ѧ�ij������ʣ���A��B��C������ͬһ��Ԫ�أ���һ������������֮����ת����ϵ��ͼ��ʾ�����ַ�Ӧ�е�H2O����ȥ����
��֪A��B��C��D����ѧ��ѧ�ij������ʣ���A��B��C������ͬһ��Ԫ�أ���һ������������֮����ת����ϵ��ͼ��ʾ�����ַ�Ӧ�е�H2O����ȥ���� ��ͼ��ʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼ��ʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺