��Ŀ����
4����һ��ɫ��������У�Ca2+��Cl-��Cu2+��CO32-��K+��Na+�еļ��֣���������ʵ�飺��1���Ѹù����������ˮ�е�һ��ɫ��Һ�Ͱ�ɫ��������2��ȡ��1����������ɫ��Һ�����AgNO3��Һ�а�ɫ�����������ټ�ϡHNO3����ȫ�������������ų�������1���ð�ɫ������һ���е�������Ca2+��CO32-��
��2���ð�ɫ������һ��û�е�������Cl-��Cu2+��
��3���ð�ɫ�����п����е�������K+��Na+����Ҫ��֤���������Ƿ�һ�����ڣ�������ɫ��Ӧ�������м��飮
���� ��1���Ѹù����������ˮ�е�һ��ɫ��Һ�Ͱ�ɫ��������Cu2+��Ӧ����Ca2+��CO32-������̼��Ƴ�����
��2��ȡ��1����������ɫ��Һ�����AgNO3��Һ�а�ɫ�����������ټ�ϡHNO3����ȫ�������������ų�����Cl-���Դ˽����⣮
��� �⣺��һ��ɫ��������У�Ca2+��Cl-��Cu2+��CO32-��K+��Na+�еļ��֣���������ʵ�飺
��1���Ѹù����������ˮ�е�һ��ɫ��Һ�Ͱ�ɫ��������Cu2+��Ӧ����Ca2+��CO32-������̼��Ƴ�����
��2��ȡ��1����������ɫ��Һ�����AgNO3��Һ�а�ɫ�����������ټ�ϡHNO3����ȫ�������������ų�����Cl-��
��1�������Ϸ�����֪һ�����е�����ΪCa2+��CO32-���ʴ�Ϊ��Ca2+��CO32-��
��2��һ��û�е�����ΪCl-��Cu2+���ʴ�Ϊ��Cl-��Cu2+��
��3������û���漰��K+��Na+������ʵ�飬����ȷ��K+��Na+�Ƿ���ڣ�������ɫ��Ӧ�����Ƿ���ڣ��ʴ�Ϊ��K+��Na+����ɫ��Ӧ��
���� ���⿼���˳������Ӽ��飬��Ŀ�Ѷ��еȣ�ע�����ճ����������ӵ����ʼ����鷽������ȷ��������ɫ�������ƣ����Ӽ���ʱ�����ų��������ӣ�ע����鷽���������ԣ�

 �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�| A�� | Ba2+��Cu2+��NO3-��SO42- | B�� | K+��Na+��SO42-��Cl- | ||
| C�� | CO32-��H+��Na+��K+ | D�� | H+��Cl-��NO3-��Ag+ |
| A�� | CO�ǻ�ԭ�� | B�� | Fe2O3����ԭ | C�� | ����������CO2 | D�� | �����û���Ӧ |
| A�� |  ȡ��ҩƷ | B�� | 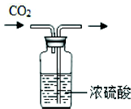 ���������̼ | C�� |  �ⶨij��Һ��pH | D�� |  �μ�Һ�� |
| A�� | ������̼ԭ�Ӳ���һ��ֱ���� | B�� | �ȶ������Һ�� | ||
| C�� | 1mol������ȫȼ������������5mol | D�� | �������ܹ�����ȡ����Ӧ |
 ��֪��A������ʯ�͵���Ҫ�л�����ԭ�ϣ������ʿ�����������һ������ʯ�ͻ�����չˮƽ��E�Ǿ��й���ζ������F��һ�ָ߾�����Ƴɶ��ְ�װ���ϣ�
��֪��A������ʯ�͵���Ҫ�л�����ԭ�ϣ������ʿ�����������һ������ʯ�ͻ�����չˮƽ��E�Ǿ��й���ζ������F��һ�ָ߾�����Ƴɶ��ְ�װ���ϣ�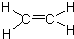 ��C������Ϊ��ȩ��
��C������Ϊ��ȩ��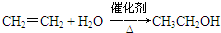 ��
�� ��
��