��Ŀ����
ʵ������Ҫ����480 mL 0��4 mol/L��NaCl��Һ�������²������裺�ٰѳ����õ�NaCl�������С�ձ��У�����������ˮ�ܽ⣬�ڰѢ�������ҺС��ת��һ���ݻ�������ƿ�У��ۼ���������ƿ�м�����ˮ��Һ���̶���1��2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����̶������У�������������ˮϴ���ձ��Ͳ�����2��3 �Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ��������ҡ�ȣ��ݽ�����ƿƿ�����������ҡ�ȡ�����д���пհף�
��1�������������ȷ˳��Ϊ ������ţ���
��2����ʵ��Ӧѡ�� mL������ƿ��Ӧ��ȡNaCl���������Ϊ ��
��3�����������ƫ�ߡ�����ƫ�͡�������Ӱ�족����
�ٳ���NaCl����ʱ������ŷ���1 g���������룩 ���ڶ���ʱ���̶��� ��
��û�н��в�������� ���ܼ�����ˮʱ���������˿̶��ߣ������ý�ͷ�ιܽ������ˮ���� ��������ƿ��ԭ������������ˮ ��
��1���٢ڢܢۢ� ��2�֣� ��2��500 ��1�֣���11��7g ��2�֣�
��3����ƫ�ͣ���ƫ�ߣ���ƫ�ͣ���ƫ�ͣ�����Ӱ�졣����1�֣�
���������������1������ʱ��һ��ɷ�Ϊ���¼������裺���㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�����������������ȷ˳��Ϊ�٢ڢܢۢݣ���2��ʵ����û��480mL������ƿ��Ҫѡȡ500mL����ƿ����ѡ�ۣ���3����Ҫ����ƽ����ҩƷ����ҩ��ȡҩƷ�����ձ��ܽ�ҩƷ���ý�ͷ�ιܶ��ݣ�����Ͳ��ȡ����ˮ���ʴ�Ϊ����ƽ��ҩ�ס��ձ�����ͷ�ιܡ���Ͳ��
��4���ܽ���塢ת����Һʱ�ò��������������н�������������ã��ʴ�Ϊ���٢ڢۢܣ���5�����ܲ�����ת��ϴ��Һ����Ҫʹ�ò������������ʴ�Ϊ��������
���㣺һ�����ʵ���Ũ����Һ�����ơ�������ѡ��

�������ʵ��ŷŲ���Ⱦ��������
| A��ȼú���� | B���ͳ����� | C������β�� | D����¯ˮ���� |
��12�֣�(ʵ����)��ͼΪ����250 mL 0��2 mol/L Na2CO3��Һ��ʾ��ͼ��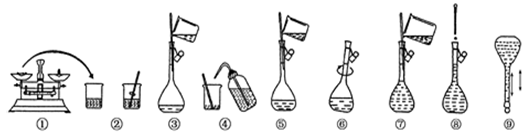
�ش��������⣺
��1�����гƵ�Na2CO3________g��ѡȡ����ƿ���______________
��2������ƿʹ��ǰ����©ˮ�ķ����� ____________________��
��3�������������������������ҺŨ���к�Ӱ�죿(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
| A��ijͬѧ�ڵڢಽ�۲�Һ��ʱ����________�� |
| B��û�н��в�������ܺ͢�________�� |
| C���ڵڢݲ�����������Һ����������ƿ�� ________�� |
| D��δ����ȴ���Ƚ���Һע������ƿ�ж��� ________�� |
�����й��������ʵ�Ӧ�ô������
| A���������費��ǿ�ᷴӦ������ʯӢ��������ʢ������� |
| B��̼�����ƾ��������ԣ�������ʳƷ���� |
| C���������ƾ���ǿ�����ԣ���������������Һ |
| D��������ˮ������Al(OH)3���壬��������ˮ�� |
�йغϽ��������ȷ����( )
| A���Ͻ�ĵ����Աȳɷֽ���ǿ |
| B���Ͻ�Ŀ���ʴ���ܶ��ܺ� |
| C���Ͻ�Ļ�еǿ�ȱȸ��ɷֽ���С |
| D�������Ͻ�ȳɷֽ����۵�ͣ�Ӳ�ȴ� |
