��Ŀ����
��12�֣�(ʵ����)��ͼΪ����250 mL 0��2 mol/L Na2CO3��Һ��ʾ��ͼ��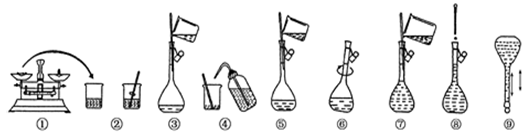
�ش��������⣺
��1�����гƵ�Na2CO3________g��ѡȡ����ƿ���______________
��2������ƿʹ��ǰ����©ˮ�ķ����� ____________________��
��3�������������������������ҺŨ���к�Ӱ�죿(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
| A��ijͬѧ�ڵڢಽ�۲�Һ��ʱ����________�� |
| B��û�н��в�������ܺ͢�________�� |
| C���ڵڢݲ�����������Һ����������ƿ�� ________�� |
| D��δ����ȴ���Ƚ���Һע������ƿ�ж��� ________�� |
��1�� 5��3 250ml (ÿ��2��)
��2��������ƿ�ڼ�����ˮ������ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף���ƿ�����������粻©ˮ����ƿ����ת180����������ٰ�ƿ��������������©ˮ������ʹ�ã�3�֣�
��3����Aƫ�ߣ�Bƫ�ͣ� Cƫ�ͣ� Dƫ�ߣ� Eƫ�͡���ÿ��1�֣�
�������������ʵ������250 mL 0��2 mol/L Na2CO3��Һ����ѡ��250ml������ƿ��������Һ���ȡNa2CO3������Ϊ��0��2mol/L��0��25L��106g/mol=5��3g��ѡȡ����ƿ�����©������Ϊ��������ƿ�ڼ�����ˮ������ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף���ƿ�����������粻©ˮ����ƿ����ת180����������ٰ�ƿ��������������©ˮ��������ƿ����ʹ�ã�����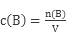 ��֪������ʱ������ࣩ���ӻᵼ��������Һ���VƫС����ᵼ������������Һ��Ũ��ƫ�ߣ�û�н���ϴ��ת�ƣ�����ܡ��ݣ�����ᵼ��n(B)��С����ᵼ��������ҺŨ��ƫ�ͣ�ת��Һ�壨����ݣ�ʱҺ�彦���ᵼ��n(B)��С����ᵼ��������ҺŨ��ƫ�ͣ�δ����ȴ���Ƚ���Һע������ƿ�ж��ݣ���Һ����������ͣ��ᵼ����Һ���VƫС����������ҺŨ��ƫ�ߣ�ҡ�Ⱥ���Һ����ڿ̶�������Ϊ����ҡ�ȵĹ����У���һ����Һ��ճ���˿̶����Ϸ��������ϣ������貹��ˮ�����Լ�ˮ��ᵼ��������ҺŨ��ƫ�͡�
��֪������ʱ������ࣩ���ӻᵼ��������Һ���VƫС����ᵼ������������Һ��Ũ��ƫ�ߣ�û�н���ϴ��ת�ƣ�����ܡ��ݣ�����ᵼ��n(B)��С����ᵼ��������ҺŨ��ƫ�ͣ�ת��Һ�壨����ݣ�ʱҺ�彦���ᵼ��n(B)��С����ᵼ��������ҺŨ��ƫ�ͣ�δ����ȴ���Ƚ���Һע������ƿ�ж��ݣ���Һ����������ͣ��ᵼ����Һ���VƫС����������ҺŨ��ƫ�ߣ�ҡ�Ⱥ���Һ����ڿ̶�������Ϊ����ҡ�ȵĹ����У���һ����Һ��ճ���˿̶����Ϸ��������ϣ������貹��ˮ�����Լ�ˮ��ᵼ��������ҺŨ��ƫ�͡�
���㣺һ�����ʵ���Ũ����Һ������ʵ�顣

Ϊ�˱������ˮ�帻Ӫ�������ҹ���ֹ���������ۺ��� ����ϴ�·�
| A��Ca | B��Cl | C��O | D��P |
��1�֣�ʵ����Ҫ��98%(�ܶ�Ϊ1.84 g��cm��3)����������3.68 mol/L������500 mL��
������3.68 mol/L�����ᣬ������������ȷ�����в�����ʹ����������ҺŨ��ƫ�͵���___��
| A����ϡ�͵�����ת��������ƿ��δϴ���ձ��Ͳ����� |
| B�����ձ��ڵ�ϡ����������ƿ��ת��ʱ�����������ʹ����ϡ���ὦ��ƿ�� |
| C���ý�ͷ�ι�������ƿ�м�ˮʱ��Һ���������ƿ�̶��ߣ���ʱ�����ý�ͷ�ιܽ�ƿ�ڶ���Һ��������ʹ��Һ��Һ����̶������� |
| D���ý�ͷ�ι�������ƿ�м���ˮʱ�����ӹ۲���Һ��Һ��������ƿ�̶������� |
12��)������������Ϊ98%���ܶ�Ϊ1.84 g��cm��3��ŨH2SO4������500 mL 0.2 mol��L��1��ϡH2SO4���ɹ�ѡ��������У��ٲ����� ����ƿ ���ձ� ��ҩ�� ����Ͳ ������ƿ ��������ƽ��
��ش��������⣺
��1�����������У�������ϡH2SO4ʱ�ò������� (�����)������ʱ��Ƿȱ�������� ��
��2�������㣬��ŨH2SO4�����Ϊ mL����ȷ��0.1����
��3�����ƹ��������²�����
| A����Һ |
| B����ȡ |
| C��ϴ�� |
| D������ |
F��ҡ��
����ȷ�IJ���˳��Ӧ�� (�����)��
��4�������ƹ����У�����������ȷ�����в����У����������ƫ�ߵ��� (�����)��
��ϴ����ȡŨH2SO4�����Ͳ������ϴ��Һת�Ƶ�����ƿ��
��ϡ�ͺ��H2SO4��Һδ����ȴ�����¾�ת�Ƶ�����ƿ��
�۶���ҡ�Ⱥ���Һ����ڱ��ߣ����ý�ͷ�ιܼ�����ˮ������
��ת��ǰ������ƿ�к�����������ˮ
�ݶ���ʱ�����ӱ���
��5������������ƿ����ȡ25.00mL��ϡ������Һ��100mL������ƿ����ˮϡ�����̶��ߡ�����������Һ��c��H+��= ��
��6��ij�о�С�������������Ͷ�������ͨ��ˮ��Һ�����Ʊ�100mL��0.4molH+����Һ����Ӧԭ����Cl2+SO2+2H2O= H2SO4+2HCl���������Ʊ��������������ģ��������״���µ����� L��
��14�֣�ʵ���ҿ����ø�����غ�Ũ���ᷴӦ��ȡ��������Ӧ�Ļ�ѧ����ʽ���£�
2KMnO4 +16HCl(Ũ) 2KCl + 2MnCl2 + 5Cl2�� +8H2O
��1���õ����ŷ��������ת�Ƶķ������Ŀ��
��2���÷�Ӧ�е��������뻹ԭ�����ʵ���֮���� ��
��3��KMnO4�������Ա�Cl2�������� ��ѡ�ǿ������������
��4���練Ӧ��ת����2mol���ӣ��������Cl2�ڱ�״�������Ϊ L��
��5��ijͬѧ����KMnO4��������100 mL0.5mol.L-1����Һ���ش��������⣺
������KMnO4��Һʱ���õ���Ҫ������������ƽ��ҩ�ס��ձ�������������Ͳ�� �� ��
��Ӧ��������ƽ��ȡKMnO4���� g��
�۲��淶��ʵ������ᵼ��ʵ���������������в�����ʵ������Ӱ��ƫС���ǣ�������ţ� ��
| A����ˮ����ʱ���ӿ̶��� |
| B������ƿ�ڱڸ���ˮ���δ���ﴦ�� |
| C���ߵ�ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ���� |
| D�����ܽ������������Һ�彦���ձ��� |
����ƿʧȥ��ǩ��Na2CO3��Һ��NaHCO3��Һ�����ǵ����ʵ���Ũ����ͬ�������Լ�������������������Һ����
| A����̪��Һ | B��BaCl2��Һ | C��NaOH��Һ | D������ʯ��ˮ |
