��Ŀ����
8��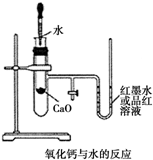 ��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�ã���ʵ������������£�
��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�ã���ʵ������������£��ٰ�ͼ��ʾ��ʵ��װ�����Ӻã�
����U�ι��ڼ���������īˮ����Ʒ����Һ������T�ιܻ�����ʹU�ι������ߵ�Һ�洦��ͬһˮƽ�棬�ٹر�T�ιܻ�����
����ʢ��1.0g�����Ƶ�С�Թ������2mL���ҵ�����ˮ���۲�����
�Իش�
��1��ʵ��ǰ������е�һ��ʵ������Ǽ��װ�õ������ԣ�
��2��ʵ���й۲쵽��������U�ι����Һ������½����ұ�������
��3��ʵ���з����Ļ�ѧ��Ӧ����ʽ��CaO+H2O�TCa��OH��2��
��4��˵��CaO��H2O��������Ca��OH��2������֮��Ĺ�ϵ��1molCaO��1molH2O�������ʹ���1molCa��OH��2��������
��5������ʵ����CaO����NaCl��ʵ�黹�ܷ�۲쵽��ͬ������ܡ�����
���� ��1��������ѹԭ���µ�ʵ������һ��Ҫ��֤װ�ò�©����
��2������ʵ������ҩƷ�������Լ����������������������ش�
��3��CaO��ˮ��Ӧ�Ļ�ѧ��Ӧ����Ca��OH��2��
��4�����ݷ�Ӧ����������������������֮��Ĵ�С��ϵ������Ӧ�������������
��5���Ȼ��ƺ�ˮ��Ϻ������ı仯�ܲ����ԣ�
��� �⣺��1����ʵ����������ѹԭ���µ�ʵ��������֣�����ʵ��֮ǰһ��Ҫ���װ�������ԣ��ʴ�Ϊ�����װ�������ԣ�
��2��CaO��ˮ��Ӧ�ų�������ʹ���Թ��п������ͣ��ڲ�ѹǿ������U�ι����Һ������½����ұ��������ʴ�Ϊ��U�ι����Һ������½����ұ�������
��3��CaO��ˮ��Ӧ�����������ƣ���Ӧ�Ļ�ѧ��Ӧ����ʽΪ��CaO+H2O�TCa��OH��2���ʴ�Ϊ��CaO+H2O�TCa��OH��2��
��4��CaO+H2O�TCa��OH��2������ʵ������֪���������ƺ�ˮ֮��ķ�Ӧ�Ƿ��ȵģ�1 mol CaO��1 mol H2O�������ʹ���1 mol Ca��OH��2��������
�ʴ�Ϊ��1 mol CaO��1 mol H2O�������ʹ���1 mol Ca��OH��2��������
��5���Ȼ��ƺ�ˮ��Ϻ������ı仯�ܲ����ԣ��Թ�������ѹǿ�������䣬��������κ����ʴ�Ϊ����
���� ���⿼����̽�����ȷ�Ӧ����ȷ�Ӧ����Ŀ�Ѷ��еȣ�ע��֪ʶ��Ǩ�ƺ�Ӧ���ǹؼ�������������ѧ���ķ�����������������ѧʵ��������


| A�� | �ͽ����Ʒ�Ӧʱ���Ѣ� | |
| B�� | �Ҵ�ȼ��ʱ���Ѣں͢� | |
| C�� | �����������£���O2��Ӧʱ���Ѣٺ͢� | |
| D�� | ��ŨH2SO4�����Ṳ��ʱ���Ѣ٣����������仯 |
| A�� | ��0.1mol•L-1��ˮ��pHΪ11��NH3•H2O?NH${\;}_{4}^{+}$+OH- | |
| B�� | ��Na�����ˮ�У��ų����壺2Na+2H2O=2NaOH+H2�� | |
| C�� | ��Ƭ����NaOH��Һ�У��������壺2Al+2OH-+2H2O=2AlO${\;}_{2}^{-}$+3H2�� | |
| D�� | ��CuCl2��Һ������ʵ�飬���ݷ��⣺CuCl2$\frac{\underline{\;���\;}}{\;}$Cu2++2Cl- |
| A�� | X��ԭ��������Y��С | B�� | X��ԭ�Ӱ뾶��Y�Ĵ� | ||
| C�� | Xԭ�ӵ�������������Y�Ĵ� | D�� | XԪ�ص�������۱�Y�Ĵ� |
 ��ʾ�ķ���ʽ�ṹ��ʽ������C6H14����CH3��2CHCH2CH2CH3��2-�����飮
��ʾ�ķ���ʽ�ṹ��ʽ������C6H14����CH3��2CHCH2CH2CH3��2-�����飮��2���������ʵĹ�ҵ�Ҵ�������װ���У����������оƾ��ơ�������ƿ�������ܡ�β�ӹܡ���ƿ��
��3��Ϊ�о�ij�л���A�������ṹ������������ʵ�飺
| ʵ�鲽�� | ���ͻ�ʵ����� |
| ��1����ȡA���� 18.0g������ʹ�������������ܶ�����ͬ������H2��45���� | ��ͨ��������գ� ?A����Է�������Ϊ��90 |
��2��A�ĺ˴Ź���������ͼ�� | ?A�к���4����ԭ�� |
| A�� | ����ĵ���ʽ | B�� | ̼-12ԭ�ӣ�612C | ||
| C�� | �����Ƶĵ���ʽ | D�� | �����ӵĽṹʾ��ͼ |

| A�� | ����װ����ʯīI��ʯīII������ | |
| B�� | ��Ԫ����װ�â��б���������װ�â��б���ԭ | |
| C�� | ����MnO2�ĵ缫��ӦʽΪ��MnO2+2H2O+2e-�TMn2++4OH? | |
| D�� | ��Ӧ�١��������ɵ�����I2ʱ������ͨ���ĵ�����֮��Ϊ1��5 |
���ᡡ
�������
�ۺ����ᡡ
��һԪ�ᡡ
�ݵ����
����
���������
| A�� | �٢ڢۢܢݢ� | B�� | �٢ܢޢ� | C�� | �٢ڢۢܢ� | D�� | �٢ۢܢݢ� |
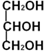 �����������ᣨCH3CH2COOH�����������ķ�Ӧ����ʽ��
�����������ᣨCH3CH2COOH�����������ķ�Ӧ����ʽ��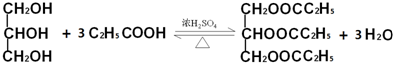
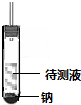 �������ݲ������Ҳ����������ȼ������ṹΪCH3CH2OH����������������ΪCH3OCH3��
�������ݲ������Ҳ����������ȼ������ṹΪCH3CH2OH����������������ΪCH3OCH3��