��Ŀ����
6����֪����A ��ʯ���ѽ�������Ҫ�ɷ֣�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ��
��2CH3CHO+O2$��_{��}^{����}$2CH3COOH
����֪����F�Ľṹ��ʽΪ

������AΪ��Ҫԭ�Ϻϳ������������߷��ӻ�����E����ϳ�·����ͼ��

�ش��������⣺
��1��A�ĵ���ʽΪ��

��2��д���������з�Ӧ�ķ�Ӧ���ͣ��ټӳɷ�Ӧ ��������ȡ������Ӧ
��3����֤D��F���еĹ����Ų��õ��Լ���̼������Һ����ɫ��ʯ����Һ������������Ϊ�����ݲ�������Һ���ɫ
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
��A��BCH2=CH2+H2O$��_{��}^{����}$C2H5OH
��B+D����������CH3COOH+CH3CH2OH
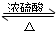 CH3COOC2H5+H2O
CH3COOC2H5+H2O��5��ʵ������ȡ���������������÷�Һ�����������������뱥��̼������Һ��
���� A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ����BΪCH3CH2OH���Ҵ���������CΪCH3CHO��CH3CHO��һ�������ɵ�DΪCH3COOH��CH3COOH��CH3CH2OH����������Ӧ����CH3COOCH2CH3����ϩ�����Ӿ۷�Ӧ���ɸ߷��ӻ�����E��EӦΪ ���ݴ˷������
���ݴ˷������
��� �⣺A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ����BΪCH3CH2OH���Ҵ���������CΪCH3CHO��CH3CHO��һ�������ɵ�DΪCH3COOH��CH3COOH��CH3CH2OH����������Ӧ����CH3COOCH2CH3����ϩ�����Ӿ۷�Ӧ���ɸ߷��ӻ�����E��EӦΪ ��
��
��1��������������֪��AΪC2H4������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2���٢ܷ�Ӧ���ͷֱ��Ǽӳɷ�Ӧ��������Ӧ��ȡ����Ӧ��
�ʴ�Ϊ���ӳɷ�Ӧ��������ȡ������Ӧ��
��3��D��F�ж������Ȼ������ܺ�̼������Һ��Ӧ�������壬����ʹ��ɫʯ����Һ����ɫ�����Կ�����̼������Һ����ɫʯ����Һ�����Ȼ��������������������ݲ�������Һ���ɫ��
�ʴ�Ϊ��̼������Һ����ɫ��ʯ����Һ�������ݲ�������Һ���ɫ��
��4����A����B�ķ�ӦΪ�Ҵ���ˮ�ļӳɷ�Ӧ����Ӧ����ʽΪCH2=CH2+H2O$��_{��}^{����}$C2H5OH��
��B+D��������������Ӧ����ʽΪCH3COOH+CH3CH2OH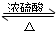 CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
�ʴ�Ϊ��CH2=CH2+H2O$��_{��}^{����}$C2H5OH��CH3COOH+CH3CH2OH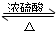 CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
��5������������ˮ�����ܣ�����ʵ������ȡ���������������÷�Һ�����������������뱥��̼������Һ��
�ʴ�Ϊ����Һ��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ����������ݷ�Ӧ�����������Ϣ�����ƶϣ���ȷ�����ż������ʹ�ϵ����Ŀ�ѶȲ���

| Ԫ�� | �ṹ�����ʵ���Ϣ |
| A | �Ƕ������У���ϡ�������⣩ԭ�Ӱ뾶����Ԫ�أ���Ԫ�ص�ij�ֺϽ���ԭ�ӷ�Ӧ�ѵĵ��ȼ� |
| B | B��Aͬ���ڣ�������������ˮ��������� |
| C | Ԫ�ص���̬�⻯�K������ˮ������������� |
| D | �Ǻ�ˮ�г��⡢��Ԫ���⺬������Ԫ�أ��䵥�ʻ���Ҳ������ˮ���������г��õ�����ɱ���� |
��1��Aԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s1��
��2��BԪ�������ڱ��е�λ��Ϊ��3���ڢ�A�壻���Ӱ뾶��BС��A������ڡ���С�ڡ�����
��3��Cԭ�ӵĵ����Ų�ͼ��
 ����ԭ�Ӻ�����3��δ�ɶԵ��ӣ�������ߵĵ���Ϊp����ϵĵ��ӣ������������Σ�
����ԭ�Ӻ�����3��δ�ɶԵ��ӣ�������ߵĵ���Ϊp����ϵĵ��ӣ������������Σ���4��Dԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p5��[Ne]3s23p5��D-�Ľṹʾ��ͼ��
 ��
��  ��ͼ��ʾ4��̼ԭ�����ϵļ��ַ�ʽ��СԲ���ʾ̼ԭ�ӣ�С����ʾ��ѧ��������̼ԭ��������Ļ�ѧ�����������ϣ�������˵����ȷ���ǣ�������
��ͼ��ʾ4��̼ԭ�����ϵļ��ַ�ʽ��СԲ���ʾ̼ԭ�ӣ�С����ʾ��ѧ��������̼ԭ��������Ļ�ѧ�����������ϣ�������˵����ȷ���ǣ�������| A�� | ͼ��������������A��C��H | |
| B�� | ͼ��C��F��������ԭ������ͬ | |
| C�� | ͼ������̼Ԫ�ص���������������C | |
| D�� | ͼ����B��Ϊͬ���칹�����E��F��H |
| A�� | ����ʽΪC4H10O����������Ʒ�Ӧ�ų��������л���������5�� | |
| B�� | ��Ӧ2H2��g��+O2��g���T2H2O��l���仯�����У����ڼ�С������S��O�� | |
| C�� | ��ϩ��1��3-����ϩ�Ļ������1 L���������������������������ӳɷ�Ӧ������1.4 L��������ԭ�����������ϩ��1��3-����ϩ�������Ϊ��3��2 | |
| D�� | ��������������Һ��������Һ�͵�ˮ���������Һ�Ƿ��Dz��ַ���ˮ�� |
| A�� | 6�� | B�� | 8�� | C�� | 12�� | D�� | 16�� |
| A�� | ������0.1mol•L-1������ҺpH=a������Һϡ�͵�ԭ�����10��pH=a+1 | |
| B�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1012��Һ�У�NH4+��Al3+��NO3-��Cl-���Դ������� | |
| C�� | CO2�����У�̼����Ϊ���Լ�����������ĺ�������IJ��غϣ�Ϊ���Է��� | |
| D�� | pH=8.0����Һ�У�CO2����Ҫ������̬ΪCO32- |
| A�� | ���ܣ�F-F��Cl-Cl��Br-Br��I-I | |
| B�� | ������F-F��Cl-Cl��Br-Br��I-I | |
| C�� | ����CO2�ǷǼ��Է��ӣ���ȩ�Ǽ��Է��� | |
| D�� | ����B3N3H6��Ϊ�ȵ����� |


 ��
�� ��
�� ��
�� ��д����ʽ��
��д����ʽ��