��Ŀ����
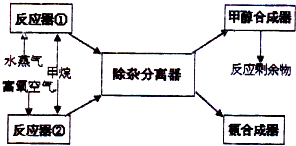 ij�������Լ���Ϊ��Ҫԭ������ȡ�ϳɼ״����ϳɰ���ԭ�ϣ�����Ҫ��ӦΪ��
ij�������Լ���Ϊ��Ҫԭ������ȡ�ϳɼ״����ϳɰ���ԭ�ϣ�����Ҫ��ӦΪ��CH4��g��+H2O��CO��g��+3H2��g���٣���Ӧ��CH4ת����Ϊ1��
2CH4��g��+O2��g����2CO��g��+4H2�ڣ���Ӧ��CH4ת����Ϊ1��
CO��g��+2H2��g����CH3OH��g������Ӧ��COת����Ϊ
| 2 |
| 3 |
�ϳɹ������£�
��ش��������⣨�����������������Ϊ��״������
��1����ֻ�÷�Ӧ������ֱ�Ӻϳɼ״��������뷴Ӧ���ٵļ����ˮ����������ֱ�Ϊ672m3��2240m3ʱ����Ӧʣ������COΪ
��2����CH4ͬʱ��H2O��g����O2��g����Ӧ����am3CH4��������ʽ��ȫ��Ӧ��������������V�ķ�ΧΪ
��3�������뷴Ӧ���ٵļ����ˮ����������ֱ�Ϊl�����3�������Ӧʣ�����У�V��H2����V��N2����V��CO��=3��1��1������뷴Ӧ���ڵĸ���������ֻ��N2��O2�������Ϊ
��4������25%�ĸ���������CH4��H2O��g����ϲ���ַ�Ӧ����Ӧ�IJ�����
| n(H2) |
| n(N2) |
���㣺��ѧ����ʽ���йؼ���
ר�⣺������
��������1�����ݷ���ʽ���й������㣬���ݲ�������ʼ������ɵ�CO��������������ٸ���n=
���㣻
��2�����ɵIJ�������Ϊ�ϳɰ���ԭ��������ֻ������ӦCH4+H2O ��g����CO+3H2����������������С��ֻ����CH4+2H2O��g����CO2+4H2����������������ݴ˼��㣻
��3�����ݷ�Ӧ�ټ��������ˮ��Ӧ���ɵ�CO���������������뷴Ӧ�����м������ΪV��������ݷ�Ӧ�ڱ�ʾ��������������Ӧ���ɵ�CO��������������������ܵ�CO������������CO��g��+2H2��g����CH3OH��g���������ĵ�CO�����������������ʾ����Ӧʣ������CO���������������ʣ���������ϵ�з��̣��ټ��㸻�����������������ʣ�����е���������������㸻������������������������������������
��4���踻������Ϊ1mol��������Ϊ0.25mol������Ϊ1mol-0.25mol=0.75mol����ˮ�����ʵ���Ϊymol�����ݷ�Ӧ�١��ڼ������Ӧ�������������ʵ��������������뵪�������ʵ���֮���з��̣��ݴ˼�����
| m |
| M |
��2�����ɵIJ�������Ϊ�ϳɰ���ԭ��������ֻ������ӦCH4+H2O ��g����CO+3H2����������������С��ֻ����CH4+2H2O��g����CO2+4H2����������������ݴ˼��㣻
��3�����ݷ�Ӧ�ټ��������ˮ��Ӧ���ɵ�CO���������������뷴Ӧ�����м������ΪV��������ݷ�Ӧ�ڱ�ʾ��������������Ӧ���ɵ�CO��������������������ܵ�CO������������CO��g��+2H2��g����CH3OH��g���������ĵ�CO�����������������ʾ����Ӧʣ������CO���������������ʣ���������ϵ�з��̣��ټ��㸻�����������������ʣ�����е���������������㸻������������������������������������
��4���踻������Ϊ1mol��������Ϊ0.25mol������Ϊ1mol-0.25mol=0.75mol����ˮ�����ʵ���Ϊymol�����ݷ�Ӧ�١��ڼ������Ӧ�������������ʵ��������������뵪�������ʵ���֮���з��̣��ݴ˼�����
���
�⣺��1������672m3������ȫ��Ӧ��Ҫˮ���������
CH4��g��+H2O=CO��g��+3H2��g��
672m3 672m3��
��ˮ������������
CH4��g��+H2O=CO��g��+3H2��g��
672m3 672m3 3��672m3
��CO�����ʵ���=
=3��104mol�������������ʵ���=
=9��104mol��
��Ӧ��COת����Ϊ
����μӷ�Ӧ��COΪ3��104mol��
=2��104mol����
CO��g��+2H2��g����CH3OH��g��
2��104mol 4��104mol
��ʣ��CO���ʵ���=3��104mol-2��104mol=1��104mol��
ʣ�����������ʵ���=9��104mol-4��104mol=5��104mol��
�ʴ�Ϊ��1��104��5��104��
��2�����ɵIJ�������Ϊ�ϳɰ���ԭ��������
ֻ������ӦCH4+H2O ��g����CO+3H2����������������С���ɷ���ʽ��֪��am3CH4��ȫ��Ӧ���������������=3am3��
ֻ����CH4+2H2O��g����CO2+4H2������������������ɷ���ʽ��֪��am3CH4��ȫ��Ӧ���������������=4am3��
�ʲ�����������V�ķ�ΧΪ��3am3��V��4am3��
�ʴ�Ϊ��3am3��V��4am3��
��3������뷴Ӧ�����м������ΪV�������
CH4��g��+H2O��CO��g��+3H2��g��
1��� 1��� 3���
2CH4��g��+O2��g����2CO��g��+4H2
V��� 0.5V��� V��� 2V���
��CO��g��+2H2��g����CH3OH��g������Ӧ��COת����Ϊ
����֪�����ĵ�COΪ
��1+V���������������Ϊ
��1+V�������
�ʣ���Ӧʣ������COΪ
��1+V�������������3���+2V���-
��1+V�����=
��5+2V�������
��
��5+2V����
��1+V��=3��1�����V=2��
�����������������=2�����0.5=1�����ʣ���е���Ϊ
��1+V�����=
��1+2��=1���������Ҫ�������������Ϊ1���+1���=2��������������������������Ϊ=
��100%=50%��
�ʴ�Ϊ��2�����50%��
��4���踻������Ϊ1mol��������Ϊ0.25mol������Ϊ1mol-0.25mol=0.75mol����ˮ�����ʵ���Ϊymol����
CH4��g��+H2O��CO��g��+3H2��g��
ymol ymol 3ymol
2CH4��g��+O2��g����2CO��g��+4H2
0.25mol 0.5mol 1mol
��3y+1��mol��0.75mol=3��1�����y=
��
��Ӧ�е�H2O��g���������������ʵ���֮��Ϊ
mol��1mol=
��
�ʴ�Ϊ��
��
CH4��g��+H2O=CO��g��+3H2��g��
672m3 672m3��
��ˮ������������
CH4��g��+H2O=CO��g��+3H2��g��
672m3 672m3 3��672m3
��CO�����ʵ���=
| 672��103L |
| 22.4L/mol |
| 3��672��103 |
| 22.4L/mol |
��Ӧ��COת����Ϊ
| 2 |
| 3 |
| 2 |
| 3 |
CO��g��+2H2��g����CH3OH��g��
2��104mol 4��104mol
��ʣ��CO���ʵ���=3��104mol-2��104mol=1��104mol��
ʣ�����������ʵ���=9��104mol-4��104mol=5��104mol��
�ʴ�Ϊ��1��104��5��104��
��2�����ɵIJ�������Ϊ�ϳɰ���ԭ��������
ֻ������ӦCH4+H2O ��g����CO+3H2����������������С���ɷ���ʽ��֪��am3CH4��ȫ��Ӧ���������������=3am3��
ֻ����CH4+2H2O��g����CO2+4H2������������������ɷ���ʽ��֪��am3CH4��ȫ��Ӧ���������������=4am3��
�ʲ�����������V�ķ�ΧΪ��3am3��V��4am3��
�ʴ�Ϊ��3am3��V��4am3��
��3������뷴Ӧ�����м������ΪV�������
CH4��g��+H2O��CO��g��+3H2��g��
1��� 1��� 3���
2CH4��g��+O2��g����2CO��g��+4H2
V��� 0.5V��� V��� 2V���
��CO��g��+2H2��g����CH3OH��g������Ӧ��COת����Ϊ
| 2 |
| 3 |
| 2 |
| 3 |
| 4 |
| 3 |
�ʣ���Ӧʣ������COΪ
| 1 |
| 3 |
| 4 |
| 3 |
| 1 |
| 3 |
��
| 1 |
| 3 |
| 1 |
| 3 |
�����������������=2�����0.5=1�����ʣ���е���Ϊ
| 1 |
| 3 |
| 1 |
| 3 |
| 1 |
| 1+1 |
�ʴ�Ϊ��2�����50%��
��4���踻������Ϊ1mol��������Ϊ0.25mol������Ϊ1mol-0.25mol=0.75mol����ˮ�����ʵ���Ϊymol����
CH4��g��+H2O��CO��g��+3H2��g��
ymol ymol 3ymol
2CH4��g��+O2��g����2CO��g��+4H2
0.25mol 0.5mol 1mol
��3y+1��mol��0.75mol=3��1�����y=
| 5 |
| 12 |
��Ӧ�е�H2O��g���������������ʵ���֮��Ϊ
| 5 |
| 12 |
| 5 |
| 12 |
�ʴ�Ϊ��
| 5 |
| 12 |
�����������Ի�ѧ��ҵ������������Ϊ���壬������ݷ���ʽ�ļ��㡢�������㣬��Ŀ���̸��ӡ���������ѧ���������ɽϸߵ�Ҫ����Ҫѧ�������������������ͼ��Ϊ�״���Ŀ���ѶȺܴ�

��ϰ��ϵ�д�
�����Ŀ
����ʱ������������Һ�������Ϻ��Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A��0.1mol?L-1��NaOH��Һ��0.1mol?L-1��H2SO3��Һ����Ϻ�����ԣ���c��H2SO3����c��SO32-�� |
| B��0.1mol?L-1��NaOH��Һ��0.1mol?L-1�Ĵ�����Һ��c��Na+����c��CH3COO-�� |
| C��pH=3�������pH=11�İ�ˮ��c��OH-����c��H+�� |
| D��0.1mol?L-1��NH4Cl��Һ��0.1mol?L-1�İ�ˮ����Ϻ�ʼ��ԣ���c��NH4+����c��Cl-�� |
�������ӷ���ʽ��ȷ���ǣ�������
| A����������ϡ���ᷴӦ��OH-+H+=H2O |
| B��С�մ������ᷴӦ��CO32-+2H+=CO2��+H2O |
| C��Fe�����ᷴӦ��2Fe+6H+=2Fe3++3H2�� |
| D������CO2ͨ��NaOH��Һ��CO2+2OH-=CO32-+H2O |
 ij��ѧʵ��С����г�������һƿijƷ��ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ʵ���ұ�NaOH��Һ������еζ��Բⶨ����ȷŨ�ȣ���ȫ��Ӧʱ������ҺpH����Ϊ9���±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����г�������һƿijƷ��ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ʵ���ұ�NaOH��Һ������еζ��Բⶨ����ȷŨ�ȣ���ȫ��Ӧʱ������ҺpH����Ϊ9���±���4�ֳ���ָʾ���ı�ɫ��Χ�� ����X2��Y�ͻ�����Z�����Ԫ�ؾ�Ϊ���ڱ���ǰ20��Ԫ�أ������ͼ�ֱ���X2��Y ��Z���й���Ϣ�Լ�����֮���ת����ϵ ����������������ȥ������ش��������⣺
����X2��Y�ͻ�����Z�����Ԫ�ؾ�Ϊ���ڱ���ǰ20��Ԫ�أ������ͼ�ֱ���X2��Y ��Z���й���Ϣ�Լ�����֮���ת����ϵ ����������������ȥ������ش��������⣺