��Ŀ����
 ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�CH3CH2OH
| Ũ���� |
| 170�� |
| ���� |
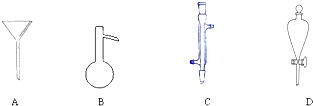
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������l40����Ӽ���ˮ�������ѣ�������������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ���й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g?cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -l30 | 9 | -1l6 |
��1���ڴ��Ƹ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����
a��������Ӧ��������b���ӿ췴Ӧ�ٶȡ���������c����ֹ�Ҵ��ӷ�������d�����ٸ�������������
��2����װ��C��Ӧ����
a��ˮ��������b��Ũ���ᡡ������������c������������Һ��������������d������̼��������Һ
��3����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ��
��4����������������δ��Ӧ��Br2�������
a��ˮ��������b������������Һ��������c���⻯����Һ��������d���Ҵ�
��5�������������������������ѣ�����
��6����Ӧ������Ϊ���ٲ���Ļӷ���������ˮ��ȴװ��D�����ֲ��ܱ�ˮ��ȴ����ԭ����
���㣺���������ȡ
ר�⣺ʵ����,�л���Ļ�ѧ���ʼ��ƶ�
��������1���Ҵ���Ũ����140��������·������Ӽ���ˮ��
��2��Ũ�������ǿ�����ԣ����������Ҵ��е�̼��
��3������1��2-���������ˮ���ܶ���Դ�С���
��4��Br2���Ժ��������Ʒ���������ԭ��Ӧ��
��5������1��2-�������������ѵķе㲻ͬ���н��
��6�����ӷ����÷�Ӧ���ȣ�
��2��Ũ�������ǿ�����ԣ����������Ҵ��е�̼��
��3������1��2-���������ˮ���ܶ���Դ�С���
��4��Br2���Ժ��������Ʒ���������ԭ��Ӧ��
��5������1��2-�������������ѵķе㲻ͬ���н��
��6�����ӷ����÷�Ӧ���ȣ�
���
�⣺��1���Ҵ���Ũ����140��������£�������������ˮ���������ѣ��ʴ�Ϊ��d��
��2��Ũ�������ǿ�����ԣ����Ҵ������ɶ�����̼����������ԭ�ɶ�����������̼�����������ܺ�����������Һ��Ӧ���ʴ�Ϊ��c��
��3��1��2-���������ˮ�����ܣ�1��2-���������ܶȱ�ˮ�ʴ�Ϊ���£�
��4��������Br2���������Ʒ�����Ӧ��2NaOH+Br2�TNaBr+NaBrO+H2O���ʴ�Ϊ��b��
��5��1��2-�������������ѵķе㲻ͬ�����߾�Ϊ�л�����ܣ�������ķ��������Ƿ��룬�ʴ�Ϊ������
��6�����ڳ����£��ӷ�����ϩ���巴Ӧʱ���ȣ�����ӷ�����ȴ�ɱ�����Ĵ����ӷ�����1��2-������������̵�9��ϵͣ����ܹ�����ȴ��
�ʴ�Ϊ��1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·������
��2��Ũ�������ǿ�����ԣ����Ҵ������ɶ�����̼����������ԭ�ɶ�����������̼�����������ܺ�����������Һ��Ӧ���ʴ�Ϊ��c��
��3��1��2-���������ˮ�����ܣ�1��2-���������ܶȱ�ˮ�ʴ�Ϊ���£�
��4��������Br2���������Ʒ�����Ӧ��2NaOH+Br2�TNaBr+NaBrO+H2O���ʴ�Ϊ��b��
��5��1��2-�������������ѵķе㲻ͬ�����߾�Ϊ�л�����ܣ�������ķ��������Ƿ��룬�ʴ�Ϊ������
��6�����ڳ����£��ӷ�����ϩ���巴Ӧʱ���ȣ�����ӷ�����ȴ�ɱ�����Ĵ����ӷ�����1��2-������������̵�9��ϵͣ����ܹ�����ȴ��
�ʴ�Ϊ��1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·������
�����������Ϊ�ۺϣ���Ҫ�������Ҵ��Ʊ�1��2-�������飬����������ʵĻ�����ѧ���ʣ��ǽ����Ĺؼ���ƽʱ��ע�������ط�Ӧ֪ʶ���Ѷ��еȣ�

��ϰ��ϵ�д�
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
�����Ŀ
һ���¶��£�ʯ�ҽ��д�������ƽ�⣺Ca��OH��2��s��?Ca2+��aq��+2OH-��aq��������һ������ʯ�ҽ��м���������ʯ�Һָ���ԭ�����¶ȣ�����˵����ȷ���ǣ�������
| A����Һ��Ca2+������Ŀ���� |
| B����Һ��c��Ca2+������ |
| C����Һ��pH���� |
| D����Һ�����ʵ������������� |
���Ͻ���м�Ӳ�����ɡ����ۡ��ྻ���ص㣬�������Ŵ���װ�β��ϣ�����������;�ص������ǣ�������
| A���������� | B���ܶ�С |
| C�������Ժ� | D��ǿ�ȸ� |


 ��
��
 �о�NO2��SO2��CO�ȴ�����Ⱦ����IJ���������������Ҫ���壮
�о�NO2��SO2��CO�ȴ�����Ⱦ����IJ���������������Ҫ���壮