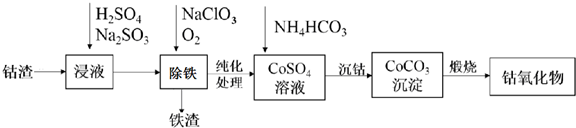
��Ŀ����
13�� ��֪Ba��AlO2��2������ˮ����ͼ��ʾ������Al2��SO4��3��Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ�������й�������ȷ���ǣ�������
��֪Ba��AlO2��2������ˮ����ͼ��ʾ������Al2��SO4��3��Һ����μ���Ba��OH��2��Һʱ�����ɳ��������ʵ���y�����Ba��OH��2�����ʵ���x�Ĺ�ϵ�������й�������ȷ���ǣ�������| A�� | A��Dʱ���������ʵ�����Al��OH��3��BaSO4�� | |
| B�� | C��Dʱ��Һ�����ӵ����ʵ�����AlO2-��Ba2+�� | |
| C�� | A��Dʱ���������ʵ�����BaSO4����С��Al��OH��3 | |
| D�� | D��Eʱ��Һ�����ӵ����ʵ�����Ba2+���ܵ���OH- |
���� A������A-B�dz�����������Ĺ��̣�����A12��SO4��3+3Ba��OH��2=2Al��OH��3��+3BaSO4����B��D��Al��OH��3�������ܽ�ת��ΪBa��AlO2��2��
B��C-D������Ӧ2Al��OH��3+Ba��OH��2=Ba��AlO2��2+4H2O��Ba��AlO2��2�����AlO2-��Ba2+��
C������A-B�dz�����������Ĺ��̣�����A12��SO4��3+3Ba��OH��2=2Al��OH��3��+3BaSO4����B��D��Al��OH��3�������ܽ�ת��ΪBa��AlO2��2��
D��D��ʱAl��OH��3����ǡ����ȫ�ܽ⣬��ʱ��Һ��ֻ����Ba��AlO2��2���������Ba��OH��2�����ʵ�������Ba��AlO2��2�����ʵ���ʱ����Һ��Ba2+��OH-������ȣ�
��� �⣺A������A-B�dz�����������Ĺ��̣�����A12��SO4��3+3Ba��OH��2=2Al��OH��3��+3BaSO4����B��D��Al��OH��3�������ܽ�ת��ΪBa��AlO2��2��Al��OH��3������ȫ��ʧ�����Գ��������ʵ�����Al��OH��3��BaSO4����A����
B��C-D������Ӧ2Al��OH��3+Ba��OH��2=Ba��AlO2��2+4H2O��1molBa��AlO2��2�����2molAlO2-��1molBa2+����AlO2-��Ba2+�࣬��B��ȷ��
C������A-B�dz�����������Ĺ��̣�����A12��SO4��3+3Ba��OH��2=2Al��OH��3��+3BaSO4����B��D��Al��OH��3�������ܽ�ת��ΪBa��AlO2��2��Al��OH��3������ȫ��ʧ�����Գ��������ʵ�����Al��OH��3��BaSO4����C����
D��D��ʱAl��OH��3����ǡ����ȫ�ܽ⣬��ʱ��Һ��ֻ����Ba��AlO2��2���������Ba��OH��2�����ʵ�������Ba��AlO2��2�����ʵ���ʱ����Һ��Ba2+��OH-������ȣ���D��ȷ��
��ѡBD��
���� ���⿼�����ӷ�Ӧ����ʽ��ͼ��ķ��������շ��������ӷ�Ӧ��ͼ���ж�Ӧ�����ӷ�ӦΪ���Ĺؼ������ط�����Ӧ�������ͼ����������ۺϿ��飬��Ŀ�Ѷ��еȣ�

| ʵ���� | HA�����ʵ���Ũ�ȣ�mol•L-1�� | NaOH�����ʵ���Ũ�ȣ�mol•L-1�� | ��Ϻ���Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.12 | 0.1 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=10 |
��2����������Һ������Ũ��c��A-����c��Na+����ȣ�����������Һ�д���3����̬ƽ�⣮
��3���ӱ���ʵ�����������û����Һ������Ũ���ɴ�С��˳����c��Na+����c��A-����c��OH-����c��H+��
��4����������ʵ�����ݣ�д���û����Һ��������ʽ�ľ�ȷ�������ʽ����c��Na+��-c��A-��=10-4-10-10mol•L-1
��5�������ӷ���ʽ���Ͷ���ҺpH=10��ԭ����A-+H2O?HA+OH-��
��ij��Ԫ�ᣨ��ѧʽ��H2B��ʾ��ˮ��Һ�����ǣ�H2B=H++HB-���� HB-?H++B2-��
�ش��������⣺
��6����0.1��mol/L��Na2B��Һ�У���������Ũ�ȹ�ϵʽ��ȷ����A��
A��c�� B2-��+c��HB-��=0.1mol/L
B��c��Na+��=2�� c�� B2-��+c��HB-��+c��H2B����
C��c��OH-��=c��H+��+c��HB-��+2c��H2B��
D��c��Na+��+c��H+��=c��OH-��+c��HB-��+c�� B2-��
| A�� | �٣��ڣ��� | B�� | �ۣ��٣��� | C�� | �ۣ��ڣ��� | D�� | �ڣ��٣��� |
| A�� | �ؽ�����ũҩ�Ȼ����ˮ����Ⱦ | |
| B�� | װ�β����еļ�ȩ�����Ȼ���ɾ�����Ⱦ | |
| C�� | CO�ᵼ��������γ� | |
| D�� | CO2�Ĵ����ŷŻ�Ӿ�����ЧӦ |
 +��2n-1��H2O
+��2n-1��H2O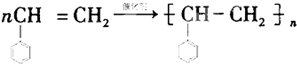
 ��NaOH�ķ�Ӧ
��NaOH�ķ�Ӧ