��Ŀ����
������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�6.02��1023�����ӱ���ԭΪ����ԭ�ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ��Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ�BԪ��ԭ�Ӻ�������������Ŀ�ȵ�һ���1����C-��AԪ�ص����Ӷ�1�����Ӳ㣬DԪ�ص�ԭ�Ӻ���ڶ���ȵ�һ���2�����ӣ��ش��������⣺
��1��A��B��C��D����Ԫ�ص����Ʒֱ��� �� �� �� ��
��2��C-�Ľṹʾ��ͼΪ ��DԪ�������ڱ��е�λ���� ��
��3��Ԫ��D�������̬�⻯��ĵ���ʽΪ ������ӵĽṹ�ص��Ǿ��� �ṹ����һ�������¸��⻯����뵥��C����ȡ����Ӧ�����������ʵ����ĸ��⻯���뵥��C��ϣ���һ�������³�ַ�Ӧ�������������ʵ��������� ���û�ѧʽ��д����
��4����ҵ��ұ������A�Ļ�ѧ����ʽΪ ��
��5����ҵ�ϳ��õ���Bұ�����۵Ľ�����д���������͵���B�ڸ����·�Ӧ�Ļ�ѧ����ʽ ���÷�Ӧ���� ��Ӧ������ȡ������ȡ�����
��6��д����ҵ����ʯӢ������D��һ�ֵ��������뵼����ϵĻ�ѧ��Ӧ����ʽ��
��7����D�ĵ�������������������ȫȼ�գ����ò���ĽṹʽΪ ��
��1��A��B��C��D����Ԫ�ص����Ʒֱ���
��2��C-�Ľṹʾ��ͼΪ
��3��Ԫ��D�������̬�⻯��ĵ���ʽΪ
��4����ҵ��ұ������A�Ļ�ѧ����ʽΪ
��5����ҵ�ϳ��õ���Bұ�����۵Ľ�����д���������͵���B�ڸ����·�Ӧ�Ļ�ѧ����ʽ
��6��д����ҵ����ʯӢ������D��һ�ֵ��������뵼����ϵĻ�ѧ��Ӧ����ʽ��
��7����D�ĵ�������������������ȫȼ�գ����ò���ĽṹʽΪ
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�6.02��1023�����ӱ���ԭΪ����ԭ�ӣ���A����Ϊ��������λ����ɵ������ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ����AO+2HCl�TACl2+H2O��M��AO��=
=40g/mol������A��Ħ������Ϊ40g/mol-16g/mol=24g/mol����Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ���������Ϊ12����AΪMgԪ�أ�BԪ��ԭ�Ӻ�������������Ŀ�ȵ�һ���1��������������Ϊ3����BΪAl��C-��AԪ�ص����Ӷ�1�����Ӳ㣬��C��������Ϊ18-1=17����CΪClԪ�أ�DԪ�ص�ԭ�Ӻ���ڶ���ȵ�һ���2�����ӣ���ڶ��������Ϊ4����DΪCԪ�أ�������Ԫ�ؼ��䵥�ʡ�����������������
| 0.4g |
| 0.01mol |
���
�⣺������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�6.02��1023�����ӱ���ԭΪ����ԭ�ӣ���A����Ϊ��������λ����ɵ������ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ����AO+2HCl�TACl2+H2O��M��AO��=
=40g/mol������A��Ħ������Ϊ40g/mol-16g/mol=24g/mol����Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ���������Ϊ12����AΪMgԪ�أ�BԪ��ԭ�Ӻ�������������Ŀ�ȵ�һ���1��������������Ϊ3����BΪAl��C-��AԪ�ص����Ӷ�1�����Ӳ㣬��C��������Ϊ18-1=17����CΪClԪ�أ�DԪ�ص�ԭ�Ӻ���ڶ���ȵ�һ���2�����ӣ���ڶ��������Ϊ4����DΪCԪ�أ�
��1������������AΪþ��BΪ����CΪ�ȣ�DΪ̼���ʴ�Ϊ��þ�������ȣ�̼��
��2��Cl��������Ϊ17�������ӽṹʾ��ͼΪ ��̼Ԫ��λ��Ԫ�����ڱ��еڶ����ڵڢ�A�壬�ʴ�Ϊ��
��̼Ԫ��λ��Ԫ�����ڱ��еڶ����ڵڢ�A�壬�ʴ�Ϊ�� ���ڶ����ڵڢ�A�壻
���ڶ����ڵڢ�A�壻
��3��D����̬�⻯��Ϊ���飬�����ʽΪ ���ռ�ṹΪ���������ͣ���������������ȡ����Ӧ������һ��ȡ��������ȡ��������ȡ��������ȡ��������HCl��������ʵ����ļ�����������ϣ���һ�������³�ַ�Ӧ�������������ʵ���������HCl��
���ռ�ṹΪ���������ͣ���������������ȡ����Ӧ������һ��ȡ��������ȡ��������ȡ��������ȡ��������HCl��������ʵ����ļ�����������ϣ���һ�������³�ַ�Ӧ�������������ʵ���������HCl��
�ʴ�Ϊ�� ���������壻HCl��
���������壻HCl��
��4����ҵ�����õ�����ڵ��Ȼ�þ��ұ��Mg����ⷴӦΪMgCl2�����ڣ�
Mg+Cl2�����ʴ�Ϊ��MgCl2�����ڣ�
Mg+Cl2����
��5����ҵ���������ȷ�Ӧ��ұ�����۵Ľ�����Al���������ķ�ӦΪFe2O3+2Al
Al2O3+3Fe���÷�Ӧ���ڷ��ȷ�Ӧ��
�ʴ�Ϊ��Fe2O3+2Al
Al2O3+3Fe�����ȣ�
��6����ҵ����ʯӢ������̼��һ�ֵ��������뵼�����Si����ѧ��Ӧ����ʽ��SiO2+2C
Si+2CO����
�ʴ�Ϊ��SiO2+2C
Si+2CO����
��7����̼�ĵ�������������������ȫȼ������CO2��������Cԭ����Oԭ��֮���γ�2�Թ��õ��Ӷԣ���ṹʽΪO=C=O���ʴ�Ϊ��O=C=O��
| 0.4g |
| 0.01mol |
��1������������AΪþ��BΪ����CΪ�ȣ�DΪ̼���ʴ�Ϊ��þ�������ȣ�̼��
��2��Cl��������Ϊ17�������ӽṹʾ��ͼΪ
 ��̼Ԫ��λ��Ԫ�����ڱ��еڶ����ڵڢ�A�壬�ʴ�Ϊ��
��̼Ԫ��λ��Ԫ�����ڱ��еڶ����ڵڢ�A�壬�ʴ�Ϊ�� ���ڶ����ڵڢ�A�壻
���ڶ����ڵڢ�A�壻��3��D����̬�⻯��Ϊ���飬�����ʽΪ
 ���ռ�ṹΪ���������ͣ���������������ȡ����Ӧ������һ��ȡ��������ȡ��������ȡ��������ȡ��������HCl��������ʵ����ļ�����������ϣ���һ�������³�ַ�Ӧ�������������ʵ���������HCl��
���ռ�ṹΪ���������ͣ���������������ȡ����Ӧ������һ��ȡ��������ȡ��������ȡ��������ȡ��������HCl��������ʵ����ļ�����������ϣ���һ�������³�ַ�Ӧ�������������ʵ���������HCl���ʴ�Ϊ��
 ���������壻HCl��
���������壻HCl����4����ҵ�����õ�����ڵ��Ȼ�þ��ұ��Mg����ⷴӦΪMgCl2�����ڣ�
| ||
| ||
��5����ҵ���������ȷ�Ӧ��ұ�����۵Ľ�����Al���������ķ�ӦΪFe2O3+2Al
| ||
�ʴ�Ϊ��Fe2O3+2Al
| ||
��6����ҵ����ʯӢ������̼��һ�ֵ��������뵼�����Si����ѧ��Ӧ����ʽ��SiO2+2C
| ||
�ʴ�Ϊ��SiO2+2C
| ||
��7����̼�ĵ�������������������ȫȼ������CO2��������Cԭ����Oԭ��֮���γ�2�Թ��õ��Ӷԣ���ṹʽΪO=C=O���ʴ�Ϊ��O=C=O��
���������⿼��ṹ����λ�ù�ϵ����Ӧ�ã��ۺ��Խ�ǿ���������ջ�ѧ���P������ұ���������ƶ�Ԫ�����ѵ㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
�����Ľṹ��ʽ���£�

�ϳ������ĵ���Ӧ�ǣ�������
��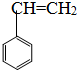 ����CH3-CH=CH-CH3�� ��CH2=CH-CH=CH2����CH��C-CH3����
����CH3-CH=CH-CH3�� ��CH2=CH-CH=CH2����CH��C-CH3����

�ϳ������ĵ���Ӧ�ǣ�������
��
 ����CH3-CH=CH-CH3�� ��CH2=CH-CH=CH2����CH��C-CH3����
����CH3-CH=CH-CH3�� ��CH2=CH-CH=CH2����CH��C-CH3����
| A���٢� | B���ܢ� | C���ۢ� | D���٢� |
����Ϊm g��ͭ��ŨH2SO4��ȫ��Ӧ���������������ΪVL����ԭ�������ǣ�������
A��
| ||
B��
| ||
C��
| ||
D��
|
������ʵ����֤��HNO2��������ʵ��ǣ�������
| A��������NaNO2��Һ��pH����7 |
| B��������0.1 mol?L-1 HNO2��Һ��pH=2.1 |
| C����HNO2��Һ������ʵ�飬���ݺܰ� |
| D��������pH=2��HNO2��Һϡ����100����pHԼΪ3.1 |
10g���н�����100mL 1mol/L�����ᷴӦ�������������ʵ��������ǣ�������
| A��Na | B��Mg | C��Fe | D��Al |
���и���������ǿ������Һ�п��Դ���������ǣ�������
| A��Ca2+ K+ CO32- Cl- |
| B��H+ Fe2+ Cl- NO3- |
| C��Na+ Ba2+ NO3- Cl- |
| D��Na+ Al3+ NO3- Cl- |
 ��ͼ��ij��ѧ��ȤС��̽����ͬ�����»�ѧ��ת��Ϊ���ܵ�װ�ã���ش��������⣺
��ͼ��ij��ѧ��ȤС��̽����ͬ�����»�ѧ��ת��Ϊ���ܵ�װ�ã���ش��������⣺