��Ŀ����
15��������N2H4�����ֳ��£���ɫҺ�壩��һ��Ӧ�ù㷺�Ļ���ԭ�ϣ�������������ϣ��ش��������⣺
��1���������ӵĵ���ʽΪ
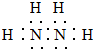 �����е��Ļ��ϼ�Ϊ-2�ۣ�
�����е��Ļ��ϼ�Ϊ-2�ۣ���2��ʵ���ҿ��ô���������Һ�백��Ӧ�Ʊ���������Ӧ�Ļ�ѧ����ʽΪ2NH3+NaClO�TN2H4+NaCl+H2O��
��3����2O2��g��+N2��g��=N2O4��l�� H1
��N2��g��+2H2��g��=N2H4��l�� H2
��O2��g��+2H2��g��=2H2O��g�� H3
��2N2H4��l��+N2O4��l��=3N2��g��+4H2O��g�� H4=-1048.9kJ/mol
������Ӧ��ЧӦ֮��Ĺ�ϵʽΪH4=2��H3-2��H2-��H1��������N2O4����Ϊ����ƽ�������Ҫԭ��Ϊ��Ӧ�����������������壮
��4������Ϊ��Ԫ�����ˮ�еĵ��뷽��ʽ�백���ƣ�������һ�����뷴Ӧ��ƽ�ⳣ��ֵΪ8.7��10-7����֪��N2H4+H+?N2H5+��K=8.7��107��KW=1.0��10-14���������������γɵ���ʽ�εĻ�ѧʽΪN2H6��HSO4��2��
��5��������һ�ֳ��õĻ�ԭ������װ������AgBr���Թ��м���������Һ���۲쵽�������ǹ�����ڣ��������ݲ��������������ڴ�����ѹ��¯ˮ�е�������ֹ��¯����ʴ��������1kg�������ɳ�ȥˮ���ܽ��O21kg����ʹ��Na2SO3����ˮ���ܽ��O2��ȣ��������ŵ���N2H4�������٣��������������ʣ���Ӧ����ΪN2��H2O������Na2SO3����Na2SO4��
���� ��1���µķ���ʽΪN2H4���ǵ�ԭ�Ӻ���ԭ���γ��ĸ����ۼ�����ԭ�Ӻ͵�ԭ��֮���γ�һ�����ۼ��γɵĹ��ۻ����Ԫ�ػ��ϼ۴�����Ϊ0���㻯�ϼۣ�
��2������������������Һ���������£��������Ʊ���ԭ�����Ȼ��ƣ�
��3����2O2��g��+N2��g���TN2O4��l����H1
��N2��g��+2H2��g���TN2H4��l����H2
��O2��g��+2H2��g���T2H2O��g����H3
�����Ȼ�ѧ����ʽ��˹���ɼ���ۡ�2-�ڡ�2-�ٵõ���2N2H4��l��+N2O4��l���T3N2��g��+4H2O��g����H4=-1048.9kJ•mol-1
��4������Ϊ��Ԫ�����ˮ�еĵ��뷽ʽ�백���ƣ�������һ�����뷽��ʽΪN2H4+H2O?N2H5++OH-��ƽ�ⳣ��Kb=$\frac{C��{N}_{2}{{H}_{5}}^{+}��C��O{H}^{-}��}{C��{N}_{2}{H}_{4}��}$=$\frac{C��{N}_{2}{{H}_{5}}^{+}��C��O{H}^{-}��}{C��{N}_{2}{H}_{4}��}$��$\frac{C��{H}^{+}��}{C��{H}^{+}��}$=K��Kw�������Ƕ�Ԫ���������������γɵ���ʽ��ΪN2H6��HSO4��2��
��5�������������������������ӱ���ԭ���ɵ�����������������ʧ����N2H4��N2-4e-��O2��4e-�������غ�����жϣ����ݹ�¯���ʵ��Լ���Ӧ�������ʽ��
��� �⣺��1���µķ���ʽΪN2H4���ǵ�ԭ�Ӻ���ԭ���γ��ĸ����ۼ�����ԭ�Ӻ͵�ԭ��֮���γ�һ�����ۼ��γɵĹ��ۻ��������ʽΪ��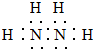 ��������Ԫ�ػ��ϼ�Ϊ+1�ۣ���Ԫ�ػ��ϼ�Ϊ-2�ۣ�
��������Ԫ�ػ��ϼ�Ϊ+1�ۣ���Ԫ�ػ��ϼ�Ϊ-2�ۣ�
�ʴ�Ϊ��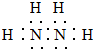 ��-2��
��-2��
��2������������������Һ���������£��������Ʊ���ԭ�����Ȼ��ƣ����ԭ���غ���ƽ��д��Ӧ�Ļ�ѧ����ʽΪ��2NH3+NaClO�TN2H4+NaCl+H2O��
�ʴ�Ϊ��2NH3+NaClO�TN2H4+NaCl+H2O��
��3����2O2��g��+N2��g���TN2O4��l����H1
��N2��g��+2H2��g���TN2H4��l����H2
��O2��g��+2H2��g���T2H2O��g����H3
�����Ȼ�ѧ����ʽ��˹���ɼ���ۡ�2-�ڡ�2-�ٵõ���2N2H4��l��+N2O4��l���T3N2��g��+4H2O��g����H4=2��H3-2��H2-��H1�����ݷ�Ӧ�ܿ�֪��������N2O4��Ӧ�ų��������Ҳ����������壬��˿���Ϊ����ƽ�����
�ʴ�Ϊ��2��H3-2��H2-��H1����Ӧ�����������������壻
��4������Ϊ��Ԫ�����ˮ�еĵ��뷽ʽ�백���ƣ�������һ�����뷽��ʽΪN2H4+H2O?N2H5++OH-��ƽ�ⳣ��Kb=$\frac{C��{N}_{2}{{H}_{5}}^{+}��C��O{H}^{-}��}{C��{N}_{2}{H}_{4}��}$=$\frac{C��{N}_{2}{{H}_{5}}^{+}��C��O{H}^{-}��}{C��{N}_{2}{H}_{4}��}$��$\frac{C��{H}^{+}��}{C��{H}^{+}��}$=K��Kw=8.7��107��1.0��10-14=8.7��10-7���ڶ������뷽��ʽΪN2H5++H2O?N2H62++OH-����������������γɵ���ʽ��ΪN2H6��HSO4��2��
�ʴ�Ϊ��8.7��10-7��N2H6��HSO4��2��
��5�������������������������ӱ���ԭ���ɵ�������-2�۵�NԪ�ر�����ΪN2����Ӧ����ʽΪ��N2H4+4AgBr=4Ag��+N2��+4HBr����˷�Ӧ��������Ϊ��������ڣ��������ݲ����������µ����������ǵ���������Թ�¯��ɸ�ʴ�����������Ʊ���������Ϊ�����ƣ������������γ���Ӱ���¯�İ�ȫʹ�ã�����������ʧ����N2H4��N2ʧȥ4e-��O2��O2-�õ�4e-������������Ħ����������32g/mol����������������������ʵ�����ͬ��������1kg�������ɳ�ȥˮ���ܽ��O21kg����ʹ��Na2SO3����ˮ���ܽ��O2��ȣ��������ŵ��������٣��������������ʣ���Ӧ����ΪN2��H2O������Na2SO3����Na2SO4��
�ʴ�Ϊ��������ڣ��������ݲ�����1��N2H4�������٣��������������ʣ���Ӧ����ΪN2��H2O������Na2SO3����Na2SO4��
���� ���⿼���˵����仯�������ʡ����ʽṹ���Ȼ�ѧ����ʽ��˹���ɼ���Ӧ�á�ƽ�ⳣ���ļ��㷽������Ҫ��������ԭ��Ӧ�ļ��㼰�������жϣ���Ŀ�Ѷ��еȣ�

 һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д����и������ʰ���ͼ��ʾת����ϵÿһ������һ��ʵ�ֵ���

�� | �� | �� | �� | |
A�� | FeCl3 | FeCl2 | Fe2O3 | Fe(OH)3 |
B�� | NO | HNO3 | NO2 | NH3 |
C�� | Cu | CuO | CuSO4 | CuCl2 |
D�� | Si | Na2SiO3 | SiO2 | SiF4 |
| A�� | ��Cu-Zn-���ᡱԭ����У����Ӵ�Zn�������ߵ���Cu���پ�����Һ�ص�Zn�γɱպϻ�· | |
| B�� | ��Al-Mg-NaOH��ԭ����У�������ǿ��Mgʧȥ���ӣ��������������� | |
| C�� | �����������Է����е�������ԭ��Ӧ������Ƴ�ԭ��� | |
| D�� | ��֪Ǧ�����ܷ�ӦΪ��Pb+PbO2+2H2SO4=2PbSO4+2H2O�����Ƹ����Ƿ�Ӧ�� Pb-2e-=Pb2+ |
 �����������ҹ�����Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⣬ֱ���ŷź�SO2���������γ����꣬Σ��������
�����������ҹ�����Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⣬ֱ���ŷź�SO2���������γ����꣬Σ����������1���û�ѧ����ʽ��ʾSO2�γ�����������ķ�Ӧ��SO2+H2O?H2SO3��2H2SO3+O2=2H2SO4����2SO2+O2?2SO3��SO3+H2O=H2SO4��2SO2+O2+2H2O=2H2SO4����
��2����ҵ����Na2SO3��Һ���������е�SO2��������ͨ��1.0mol•L-1��Na2SO3��Һ����ҺpH���ϼ�С������ҺpHԼΪ6ʱ������SO2�����������½���Ӧ�������ռ���
�ٴ�ʱ��Һ��c��SO32-����Ũ����0.2mol•L-1������Һ��c��HSO3-����1.6mol•L-1��
����pHԼΪ6�����ռ���ͨ��������O2���ɽ����е�NaHSO3ת��Ϊ�������ʣ���Ӧ�Ļ�ѧ����ʽ��2NaHSO3+O2�TNa2SO4+H2SO4��
��ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ��ģ��ʵ�����պ���������ʵ������ͼ��ʾ�����ͺ��������¶ȣ������٣������������SO2������Ч�ʣ�
��3�������ֿ��ŵ�Na2SO3ҩƷ�Ѳ��ֱ������������û�ѧС��������֪Ũ�ȵ�����KMnO4��Һ��ȷ���京�������岽�����£�
����i����ȡ��Ʒ1.000g��
����ii������Ʒ�ܽ����ȫת�Ƶ�250mL����ƿ�У����ݣ����ҡ�ȣ�
����iii����ȡ25.00mL��Ʒ��Һ��250mL��ƿ�У���0.01000mol•L-1 KMnO4����Һ�ζ����յ㣮
�����������������ظ�2�Σ�
��д������iii��������Ӧ�����ӷ���ʽ2MnO4-+5SO32-+6H+=2Mn2++5SO42-+3H2O��
��������0.01000mol•L-1 KMnO4��Һʱ�����Ӷ��ݣ������ղ��ҩƷ��Na2SO3�ĺ���ƫ���ƫ����ƫС������Ӱ�족����
��ijͬѧ����������������еζ�ʵ�飨�гֲ�����ȥ����������������AC ������ĸ����

�ܵζ�������±���ʾ��
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.20 | 20.20 |
 ������������Ϊ���͵IJ;�ԭ�ϣ�����������������������������£���Ѹ�ٷֽ�Ϊ�����ʣ����Խ��⣮�����й��������͵Ŀɽ������ϵ�������ȷ���ǣ�������
������������Ϊ���͵IJ;�ԭ�ϣ�����������������������������£���Ѹ�ٷֽ�Ϊ�����ʣ����Խ��⣮�����й��������͵Ŀɽ������ϵ�������ȷ���ǣ�������| A�� | ����������һ�ִ����� | |
| B�� | �����������еľۺϷ�ʽ��۱���ϩ���� | |
| C�� | ������һ�����߷��Ӳ��� | |
| D�� | ����Է�������Ϊ72 |
| A�� | $\frac{1000�ئͦ�}{36.5}$mol/L | B�� | $\frac{1000�ͦ�}{36.5+22400}$mol/L | ||
| C�� | $\frac{�ئ�}{22.4��V+1��}$mol/L | D�� | $\frac{1000�ͦ�}{36.5V+22400}$mol/L |
 ij����С���о��������������������£��ı���ʼ�������ʵ���[��n��H2����ʾ]��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol ��Ӧ��Ӱ�죬ʵ�����ɱ�ʾ����ͼ��ʾ�Ĺ��ɣ�T��ʾ�¶ȣ�n��ʾ���ʵ�������
ij����С���о��������������������£��ı���ʼ�������ʵ���[��n��H2����ʾ]��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol ��Ӧ��Ӱ�죬ʵ�����ɱ�ʾ����ͼ��ʾ�Ĺ��ɣ�T��ʾ�¶ȣ�n��ʾ���ʵ�������