��Ŀ����
9��2015��10���й�ҩѧ�����������������أ�һ����������ű����ҩ������ŵ��������ҽѧ���������أ�C15H22O5���Ľṹ��ͼ1��ʾ����ش��������⣺
��1����������ص�����Ԫ�ص縺���ɴ�С������O��C��H���ڻ�̬Oԭ���У��������3�������෴�ĵ��ӣ�
��2�����й��������ص�˵����ȷ����a������ţ���
a���������мȴ��ڼ��Լ��ִ��ڷǼ��Լ�
b���������ط����У�����̼ԭ�Ӿ�����ͬһƽ��
c��ͼ�����ֱ�ʶ�����̼ԭ�Ӿ�ֻ�ԦҼ�������ԭ�ӳɼ�
��3����ȷ�������ؽṹ�Ĺ����У��ɲ���NaBH4��Ϊ��ԭ�������Ʊ�����Ϊ��4NaH+B��OCH3��3��NaBH4+3CH3ONa
��NaHΪ���Ӿ��壬ͼ2��NaH�����ṹ����NaH�������λ����6���������ⳤΪa����Naԭ�Ӽ���С�˼��Ϊ$\frac{\sqrt{2}}{2}a$��
��B��OCH3��3��B���õ��ӻ�������sp2��д��������B��OCH3��3������ͬ�ռ乹�͵ķ��ӻ�����SO3��CO32-��
��NaBH4�ṹ��ͼ3��ʾ���ṹ�д��ڵ������������Ӽ�����λ�������ۼ���
���� ��1����������ص�����Ԫ��ΪH��C��O���ǽ�����Խǿ�縺��Խ���ڻ�̬Oԭ���У�������8�ĵ��ӣ�������3������гɶԵ��ӣ�
��2��a���������д���O-O����C-C����Ϊ�Ǽ��Լ���C-O��C-H��Ϊ���Լ���
b�����б���̼ԭ�ӣ����������Ľṹ��
c��C=O�����Цм���
��3����NaHΪ���Ӿ��壬NaH������ÿ����������Χ��6���⸺���ӣ��������ⳤΪa�����ھ�����С��������Խ��ߵ�Naԭ�Ӽ����С��
��B��OCH3��3��B��3��Oԭ�ӳɼ�����B��OCH3��3����ͬ�ռ乹��ƽ�������Σ�
��NaBH4�������Ӽ�����λ�����ۼ���
��� �⣺��1����������ص�����Ԫ��ΪH��C��O��Ԫ�صķǽ�����Խǿ���縺��Խǿ���ǽ����ԣ�O��C��H����H��C��O����Ԫ�صĵ縺���ɴ�С��˳����O��C��H���ڻ�̬Oԭ���У�������8�ĵ��ӣ���������Ų�ʽΪ1s22s2p4��������3������гɶԵ��ӣ���������3�������෴�ĵ��ӣ�
�ʴ�Ϊ��O��C��H��3��
��2��a���������д���O-O����C-C����Ϊ�Ǽ��Լ���C-O��C-H��Ϊ���Լ�����a��ȷ��
b�����б���̼ԭ�ӣ����������Ľṹ��������̼ԭ�Ӳ�����ͬһƽ�棬��b����
c��C=O�����Цм�����c����
�ʴ�Ϊ��a��
��3����NaHΪ���Ӿ��壬NaH������ÿ����������Χ��6���⸺���ӣ��������ⳤΪa�����ھ�����С��������Խ��ߵ�Naԭ�Ӽ����С����Naԭ�Ӽ���С�˼��Ϊ$\frac{\sqrt{2}}{2}a$��
�ʴ�Ϊ�����ӣ� 6��$\frac{\sqrt{2}}{2}a$��
��B��OCH3��3��B��3��Oԭ�ӳɼ���Ϊsp2�ӻ���B��OCH3��3����ͬ�ռ乹��ƽ�������Σ���B��OCH3��3������ͬ�ռ乹�͵ķ��ӻ����ӿ�ΪSO3��CO32-��
�ʴ�Ϊ��sp2��SO3��CO32-��
��Bԭ�Ӻ��������3�����ӣ�NaBH4�������Ӽ�����λ�����ۼ����ʴ�Ϊ�����Ӽ�����λ�������ۼ���
���� ���⿼���Ϊ�ۺϣ��漰�縺�ԡ���������Ų�����ѧ�����ȵ����塢�����ļ����֪ʶ��Ϊ�߿��������ͺ�Ƶ���㣬������ѧ���ķ��������������Ŀ��飬�ѶȲ���

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�| A�� | 6�� | B�� | 8�� | C�� | 10�� | D�� | 12�� |

| A�� | Al2O3��SiCl4��Ϊ���ۻ����� | |
| B�� | ���������о��漰������ԭ��Ӧ | |
| C�� | ʯӢ�������ƹ��ά������Si�������뵼����� | |
| D�� | ��ͭ��ұ��ͭʱ������SO2�������������ᣬFeO������ұ���� |
| A�� | ������Ϊ0.1NA ��N2 ��NH3 ������壬ԭ�Ӽ京�еĹ��õ��Ӷ���ĿΪ0.3NA | |
| B�� | 2 mol SO2 ��1 mol O2 ��һ�������³�ַ�Ӧ�����û������ķ���������2NA | |
| C�� | 1.5 mol NO2 ������ˮ��Ӧ��ת�Ƶĵ�����Ϊ1.5NA | |
| D�� | ���������£�������ΪNA ��CO��N2�����������Ϊ28 g |
| A�� | Na202������H20��Ӧ���ɱ����11.2L 02��ת�Ƶ��ӵ���ĿΪ2NA | |
| B�� | ��״���£�2.24L S03������������Ϊ4NA | |
| C�� | �ö��Ե缫���CuS04��Һ���������0.1mol Cu��0H��2��ʹ��Һ��ԭ�����·��ת�Ƶ��ӵ���ĿΪ0.2NA | |
| D�� | 0���101kp�������£�1.12L������ȫȼ�����ɵ�ˮ������Ϊ0.1NA |



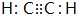 ��D�����ʽCH2=CH2��
��D�����ʽCH2=CH2�� +Br2 $\stackrel{FeBr_{3}}{��}$
+Br2 $\stackrel{FeBr_{3}}{��}$ +HBr���䷴Ӧ����Ϊȡ����Ӧ��B��C�Ļ�ѧ����ʽnCH2=CHCl$\stackrel{����}{��}$
+HBr���䷴Ӧ����Ϊȡ����Ӧ��B��C�Ļ�ѧ����ʽnCH2=CHCl$\stackrel{����}{��}$ ���䷴Ӧ����Ϊ�Ӿ۷�Ӧ��
���䷴Ӧ����Ϊ�Ӿ۷�Ӧ��

 ��
�� ��
�� ��
�� ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺